
ÄKTA ready™ Chromatography Systems from Cytiva
In the current landscape of biopharmaceuticals, single-use technologies are being increasingly incorporated into bioprocessing workflows. They offer several benefits such as enhanced manufacturing flexibility, reduced risk of contamination, and decreased downtime that can streamline process scale-up and manufacturing. These benefits align with the goals of improving efficiency, product safety, and accelerating time-to-market for novel therapeutics while being adaptable to the evolving needs of the industry.
The family of ÄKTA ready™ chromatography systems from Cytiva are a premiere example of how single-use technologies can enhance flexibility and expedite scale-up and GMP manufacturing bioprocessing workflows. These systems leverage single use disposable flow paths and ReadyToProcess™ prepacked columns that support a wide range of purification workflows.
ÄKTA ready™ systems provide the following benefits:
- The fully disposable flow path is easy and quick to change to improve process economy, productivity, and enable easy campaign changeover
- Achieve production capacity faster with simplified equipment installation and validation
- Eliminate the need for cleaning method development and validation, and minimize cross-contamination risk between products/batches
- Extensive product documentation ensures regulatory compliance and implementation in GMP environment
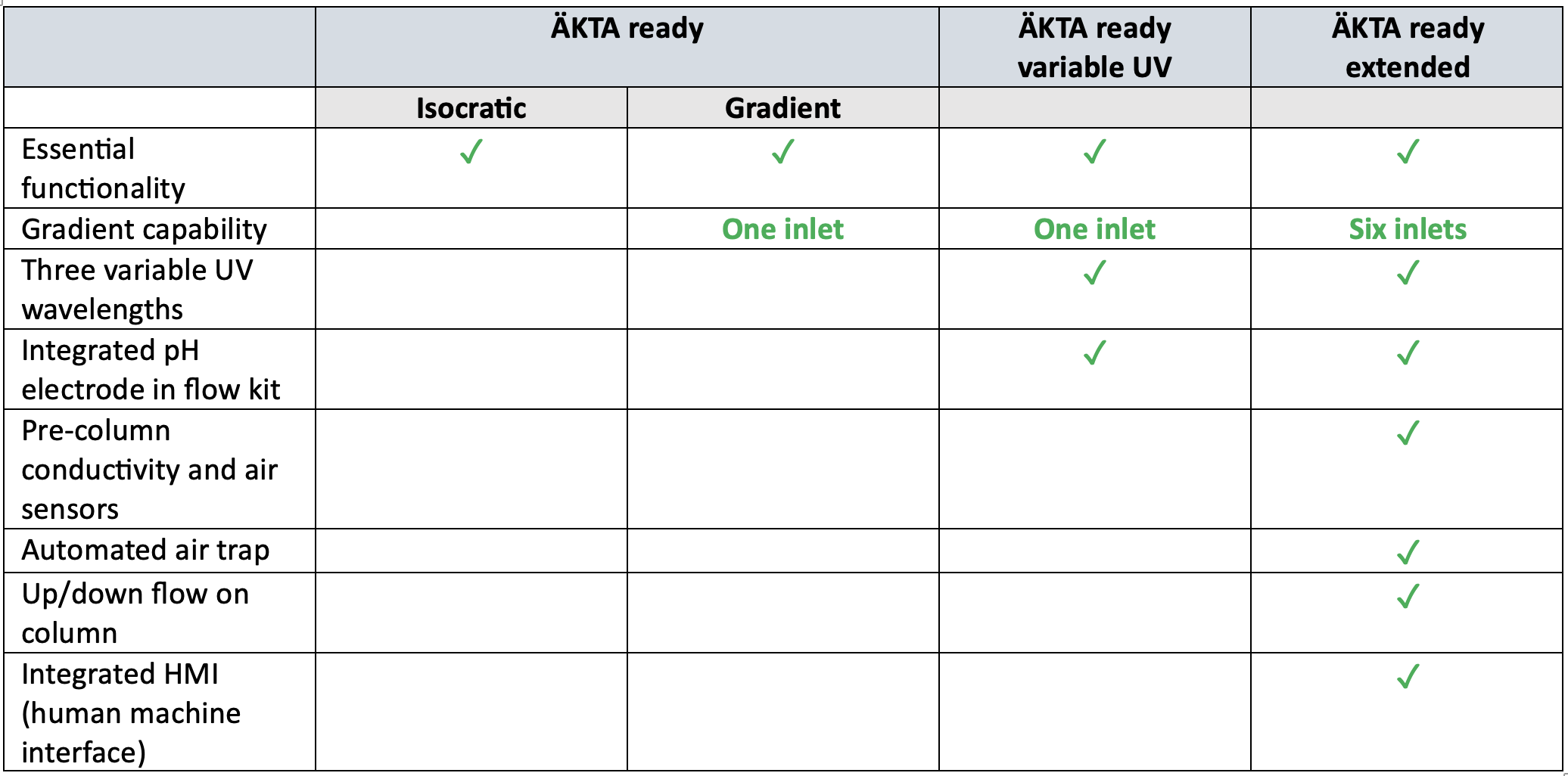
ÄKTA ready™ single use chromatography systems are available in four standard configurations (see table 1): isocratic or gradient, variable UV, and extended chromatography systems.
- ÄKTA ready™ system (isocratic and gradient capability) provides essential functionality in chromatography operations with isocratic or gradient capability. Typical applications include mAbs and recombinant protein purifications.
- The ÄKTA ready™ variable UV system includes gradient capability, three variable UV wavelengths, and an integrated pH electrode in the flow kit. Typical applications include the separation of full and empty adeno-associated virus (AAV) capsids, and nucleic acids such as plasmids and mRNA.
- The ÄKTA ready™ extended system provides additional functionalities compared to ÄKTA ready™ variable UV system.
Other configurations of the system are also available upon request.
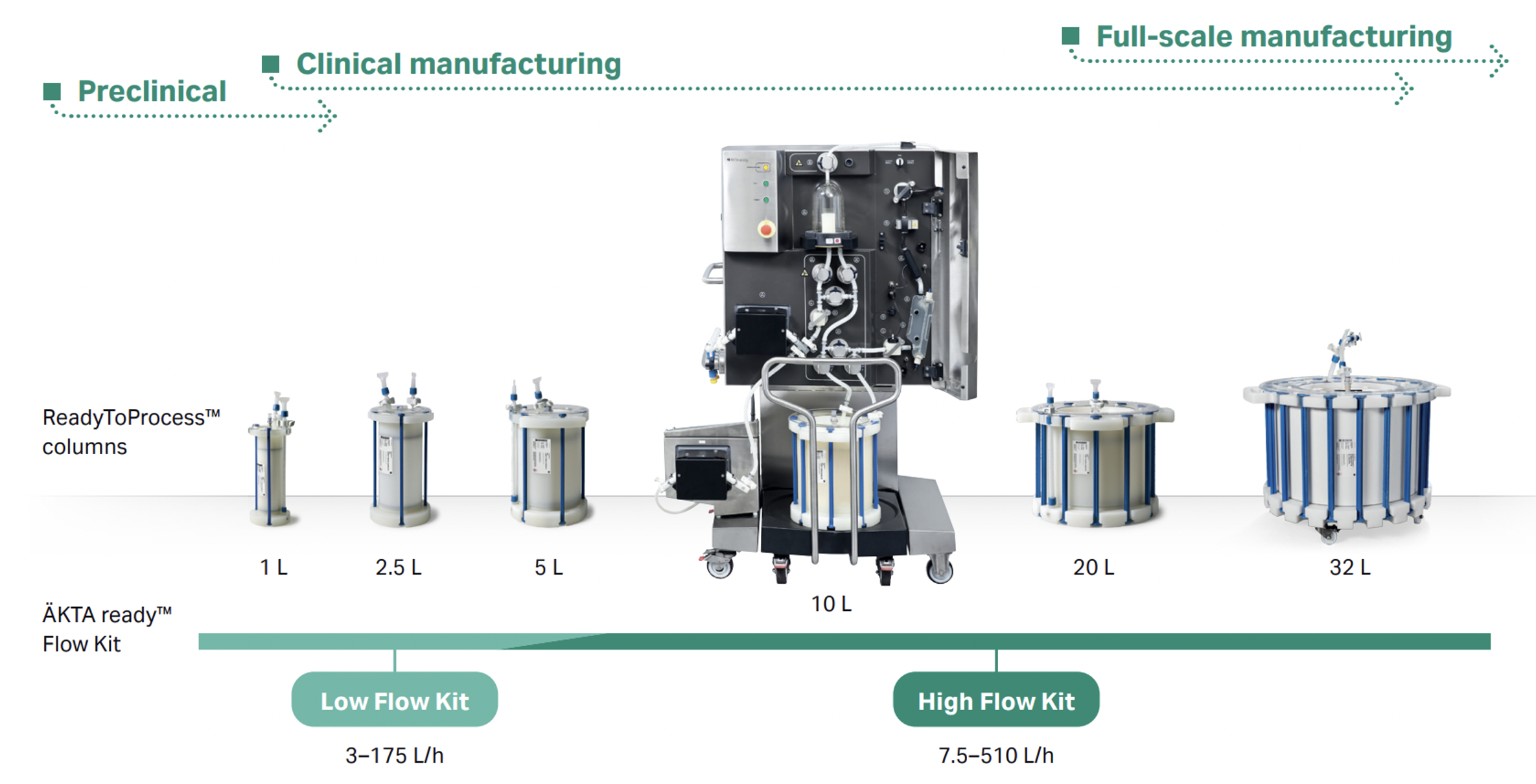
All the systems are controlled by intuitive UNICORN™ software or DeltaV™ software and come complete with an installation guide and documentation for a suite of Flow Kits and columns. Single use, disposable Low Flow and High Flow Kits are available for all system versions to accommodate a broad range of flow rates from 3 L/h to 510 L/h. This flexibility allows for optimization and adaptation to different process requirements to ensure efficient chromatographic separations.
The ÄKTA ready™ systems are designed for process scale-up and small-scale production. Methods developed at the laboratory scale can be easily transferred to the ÄKTA ready™ system and further scaled up to the ÄKTA ready™ XL system using the UNICORN™ control software, which is compatible across ÄKTA™ systems for easy method transfer.

Flow Kits and Columns
ÄKTA ready™ single use flow kits are made of high-quality materials and manufactured in controlled conditions and packed in a clean room environment (class ISO 7) using validated protocols. They are chemically resistant to reagents commonly used in protein purification processes including adsorption, elution, and washing buffers as well as regeneration and cleaning solutions. These disposable units simplify flow path exchange on ÄKTA ready™ systems, eliminating cleaning requirements, minimizing cross-contamination risk, and reducing downtime to enhance economy and productivity.
ReadyToProcess™ columns are prequalified, pre-sanitized, and prepacked with BioProcess™ chromatography resins and ready for use. Available in various diameters, the consistency in column geometry allows for convenient scaling from < 1 L (80 mm diameter) to > 57 L columns (600 mm diameter), making them suitable for pre-clinical, clinical and full-scale GMP manufacturing. With applications ranging from protein and monoclonal antibody purification in capture and polishing steps, to endotoxin removal, DNA purification, plasmid isolation, and virus separation, these closed units eliminate the need for time-consuming steps such as column packing, preparation and validation procedures.
Cytiva’s ÄKTA ready™ chromatography systems, with disposable flow paths and prepacked columns, effectively streamline chromatography unit operations to deliver flexibility, speed, and efficiency in n biopharmaceutical and bioprocessing applications.
