
Automated Buffer Preparation – Increasing Production Capacity While Maintaining Footprint
Buffer preparation requires extensive footprint and is also resource-intensive. In fact, it is known to be one of the most resource-intensive activities in biomanufacturing as large volumes of buffers and process liquids are often required as production capacity increases. Thus the question exists how can buffer preparation be more efficient and not require footprint expansion. Is there a way to increase manufacturing capacity without increasing footprint and time spent on buffers?
One possible solution is automating buffer preparation and I am pleased to share the following guest blog that discusses the advantages and addresses concerns about moving from manual to automated buffer preparation. I was fortunate to be able to interview the author about his article and have provided the transcript of our conversation following the guest blog.
Can you trust a machine for downstream bioprocess buffer preparation?
There is a limit to how many stainless-steel tanks you can squeeze into a biomanufacturing facility. Is automated buffer preparation a solution to the dilemma of balancing increased production capacity against limited footprint expansion alternatives?
Did you know that seven out of ten downstream equipment types with longest occupancy duration are buffer related? In other words, buffer management is definitely worth exploring when looking for ways to intensify your process.
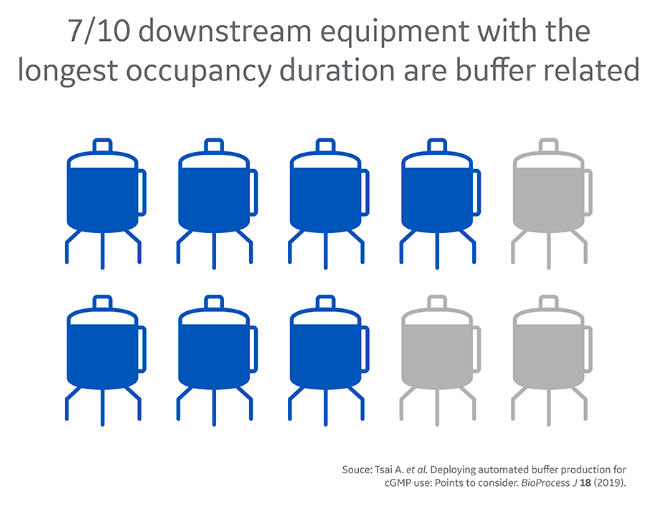
Traditionally, buffer management has received little focus in process intensification even though it can be very labor-, space-, time-, and material-intensive. Tank farms seem to be ever growing in biomanufacturing facilities, but there are available alternatives that can intensify buffer preparation.
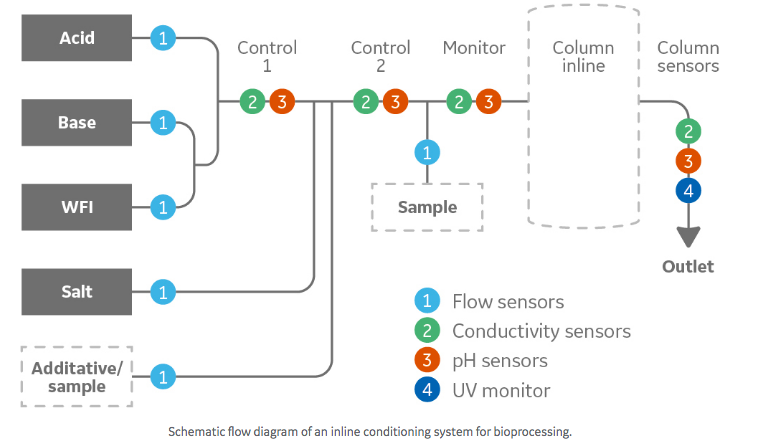
You can reduce the size and number of tanks by introducing automated just-in-time (JIT) at point-of-use (POU) solutions. Examples of such solutions are inline buffer dilution (ILD) and inline conditioning (IC). But can you trust a machine to deliver the same results as more manual buffer preparation methods?
Going from manual to automated buffer preparation
When adapting a new technology to a GMP environment, it is essential to demonstrate its benefits compared to existing methodology. In this case, compared to manual buffer preparation. New processes must also be accepted as equivalent to the existing practices to satisfy quality and regulatory requirements.
To do this, it is important to identify the critical operating parameters for buffers that make the comparison possible, revealing the potential variability. In buffer preparation, these parameters are pH and conductivity.
How does inline conditioning compare to manual buffer management?
In a recent publication, Tsai et al evaluated JIT and POU buffer delivery using solution concentrates and inline conditioning. In that study, an acetate buffer system was used in a set of experiments based on a cubic centered face (CCF) design with three factors: pH (3.7 to 5.7), buffer concentration (20 to 100 mM) and salt concentration (0 to 250 mM).
The impact of control mode was also explored. Two sets of experiments were conducted using different control modes: Recipe and Flow and pH-Flow. Recipe and Flow is a flow feedback with a calculated recipe and is essentially equivalent to ILD. pH-Flow is one of the main control modes of IC. It is a combination of flow and pH feedback, where the former is used to maintain the buffer and salt concentration.
Buffer capacity and pump flow rate are critical operating parameters
Buffer capacity and pump flow rate were identified as the critical operating parameters in controlling buffer pH when using IC. Robustness was maximized when making a buffer with high buffer capacity while operating the pumps within the flow rate specifications.
These results are aligned with the wider industry understanding that buffer preparation is dependent on how well a solution is designed around the pKa of the buffer system and on accurate measurement and delivery of individual components by the system pumps.
The control mode comparison also showed that both control modes gave similar results (within ±0.1 pH units from target with one exception [0.15]). However, the pH-Flow control mode was closer to the target (within ±0.05 pH units), indicating that if the release specification is tighter, pH-Flow feedback control would be required when using IC.
Reliable buffer conductivity prediction
Conductivity data was analyzed with respect to a typical release specification showing that when operating within the pump specification limits, each prepared buffer met the ±10% of target requirements consistently regardless of the control mode. The chemical equilibria framework predicts buffer conductivity with good agreement with actual conductivity measurements.
Can you switch to automated buffer preparation?
The study results show that automated buffer management solutions like inline conditioning can be implemented in a biomanufacturing process if the critical operating parameters are kept under control.
Manual buffer preparation still dominates the biomanufacturing industry, but major advantages can be achieved within facility use and labor requirements by marrying hardware, software algorithms, and chemistry for automated buffer preparation.
Explore available solutions for automated large-scale bioprocess buffer management.
References
Interview with Enrique Carredano, Applications Specialist GE Healthcare
Why does buffer management get overlooked when considering process intensification, considering it is so resource intensive?
This is because how things are done traditionally with the critical process parameters (CPPs) determined at lab scale where buffer volumes have no importance as compared to traditional responses like yield and purity. Process development determines what buffers, volumes and flowrates (critical process parameters) that are necessary to obtain the purity and yield (critical quality attributes) of the products. At lab scale however, when the CPP’s are determined volumes are not an issue. One can prepare 5 L of buffer quite easily. It is first after scale up that one realizes that these volumes become tens of thousands of liters. So one could say that the reason lies in the poor communication between the PD teams and the Manufacturing teams and that the hurdles of the Manufacturing teams are not part of the priorities of the PD teams. In a better world the manufacturing cost mitigation (and not only costs but knowledge of the complete working situation at the manufacturing level) should have as high importance as purity and yield during PD so that the PD people could think of a strategy to reduce those costs and hurdles.
What are the biggest benefits in moving from manual to automated buffer preparation, time, footprint, quality, etc.
All of them and they may go hand in hand since given a certain volume of resources one can prepare for more batches if basic stock solutions are prepared separately which reduces time. Quality cannot be compromised but with automated the time needed to obtain the same quality is less.
What kind of manufacturing scenarios fit particularly well with an automated buffer preparation process?
Those where several buffers can be made from the same set of stock solutions and where the number of stock solutions is less than the total number of buffers.
For people who are considering switching from manual to automated, what are the key considerations?
The automation has to be robust so that the quality of the process is guaranteed. Training is needed not only the operators and the people who will write and run the methods. It is also important to test the methods. Another important detail is to understand how the pH meters work in the liquid stream of the system and the sequence of the steps that are needed for the process to avoid unnecessary systematic errors.
What kind of support is available to companies considering switching their buffer prep process?
Demos, where the customers travel to our sites in Sweden and China to see how buffer production from different buffer systems can be automated, are constantly arranged. There are Fast Trak courses for chromatography, scale up and UNICORN programming. The special instructions that correspond to the buffer preparation part are thoroughly explained during FAT and in the operator instructions. Questions and issues can be sent to our scientific support and eventually escalated to case management where they are highly prioritized.
