Biopharmaceutical Manufacturing: Strategies for Navigating Raw Material and Process Changes

Change in inevitable, especially in biopharmaceutical manufacturing. The biopharmaceutical industry is constantly evolving with advancements in processing technologies and raw materials providing continuous improvement opportunities for manufacturers to increase efficiency, reduce costs, and mitigate risks while maintain product quality and safety across a product’s lifecycle. That said, raw materials like chromatography resins used in downstream biopharmaceutical manufacturing of regulated products are subject to strict oversight. Any changes made to the manufacturing process must be carefully evaluated using both science- and risk-based approaches and reported to regulatory authorities to ensure the safety and quality of the final product is not affected.
A white paper was recently published by Purolite, a global manufacturer of resins for the biopharmaceutical, pharmaceutical, and other industries. The publication titled “Resin Sourcing” discusses the regulatory pathways for introducing process improvements and/or alternate sources of chromatography resins into manufacturing workflows. The authors focus on regulatory and technical considerations to implement a change to a chromatography resin and provide insight into additional variables that merit further consideration such as material and supplier capabilities. The white paper also provides selected case studies outlining the approaches used by various companies in the industry to implement manufacturing changes. Here, we will summarize some of the key concepts from the publication.
Regulatory and Technical Considerations
Risk Assessment
The first step when considering a change is to understand the associated risks with respect to quality, safety and cost. Ultimately, changes made to a process must still produce a product that is comparable to the original process. Risk assessment should be used to determine the effect that proposed changes could have on the identity, strength, quality, purity, and potency of a drug or biological product. The authors note that while upstream changes in biopharmaceutical manufacturing tend to have a greater impact on the final product than downstream changes, altering downstream parameters can impact the size and structural variation of the product and process-related impurities, which may affect its safety and efficacy.
Change Reporting
For change reporting, the FDA utilizes a three tier-based classification system based on the seriousness of the change as outlined below:
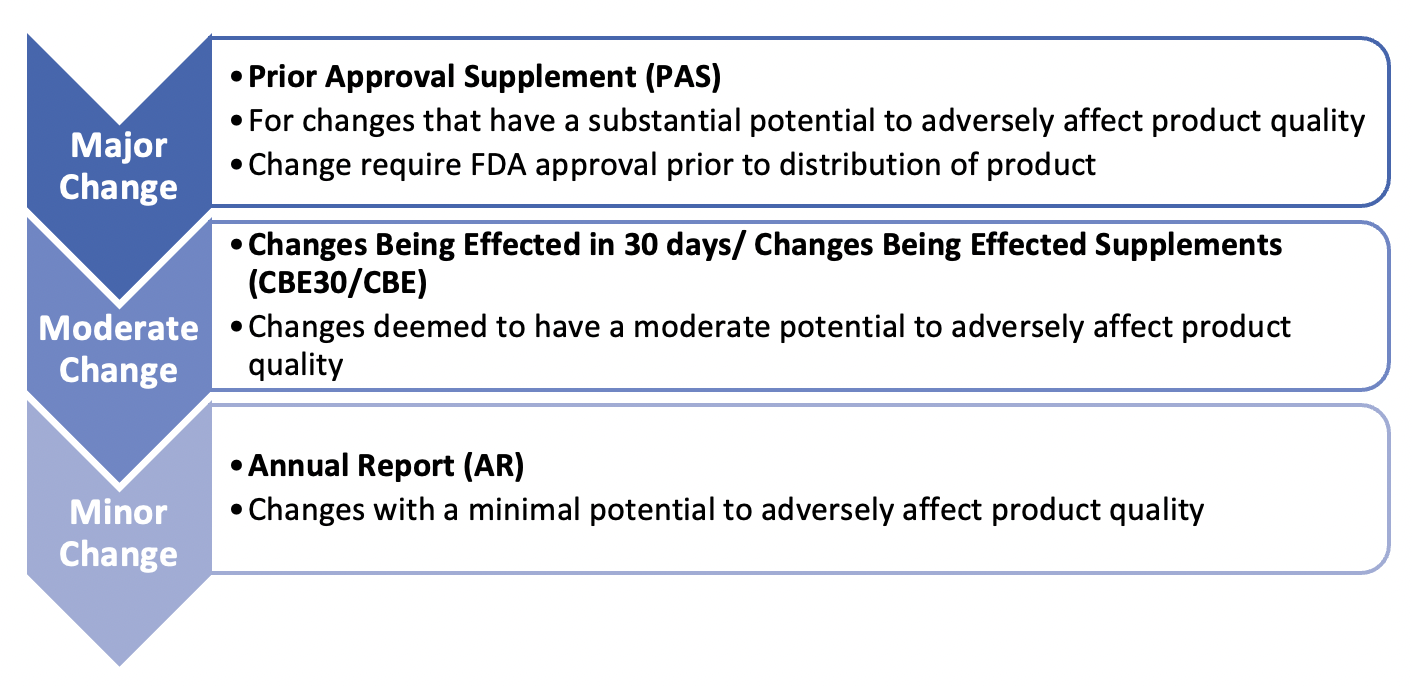
Under this system, a resin change would typically be viewed as requiring a PAS but based on the extent of the resin change (i.e., like-for-like, a minor change that is still functionally similar, or a different modality altogether) and through discussion with the appropriate regulatory authorities, the reporting category could be relaxed.
The EMA also adopts a tiered approach where type II denotes a major change, type IB are minor variations, and finally type IA, IAIN are changes where minimal or no impact is expected.
Change Assessment and Implementation
Proposed changes must be assessed, documented, and justified to demonstrate that a company has sufficient knowledge to manage the change’s impact. Documentation of a potential change must be approved by the regulatory authorities before implementation. For a PAS, CBE30 or CBE, documentation must include details such as a description of the change, the products involved, the manufacturing site or area affected, method and studies performed (comparability protocol), validation protocols and data, and a reference list of relevant standard operating procedures.
The comparability protocol is an important part of the change assessment and implementation process. It includes a detailed plan for testing and comparing the proposed change to the original process or material and its impact on the product identity, strength, quality, purity, and potency. The protocol typically includes a description of the changes, the rationale for the changes, the testing and analysis methods used to assess comparability, and the acceptance criteria used to determine whether the changes have been successfully implemented. If any differences are identified, appropriate measures can be taken to address them before implementing the change. Submitting a comparability protocol with the original application or as part of a PAS can provide an applicant with an agreed-upon plan for implementing specified changes to streamline the regulatory approval process.
Post-Approval Change Management Protocol (PACMP)
Once the proposed change has been approved, the post-approval change management protocol (PACMP) is initiated, which outlines the steps required to implement the change and ensure that the drug or biological product remains safe and effective. It includes details on the testing, documentation, control strategy to manage risks, and notification procedures that need to be followed, as well as any additional studies or data that may need to be provided to regulatory authorities.
Regulators recommend utilizing Quality by Design (QbD) approach to PACMP. QbD is a systematic and proactive approach to process development and control, which aims to ensure that a product is consistently manufactured to meet its intended quality attributes. In particular, ICH Q12 guidelines promote the use of QbD, design space, and continuous process verification to support post-approval changes. It also provides guidance on the development and implementation of a Product Lifecycle Management (PLCM) approach, which involves a coordinated and proactive management of post-approval changes across all stages of the product lifecycle.
Establishing a design space using QbD principles defines the range of process parameters that will consistently result in a drug product meeting its predefined critical quality attributes (CQAs). Manufacturers can make changes to critical process parameters (CPPs) to maintain CQAs within these limits, allowing for more flexibility in making post-approval changes without needing to submit a new application or undergo a full validation process.
The authors reference a case study published in 2008 by subject matter experts that provides comprehensive guidelines for changes to the affinity capture step (Protein A) of a hypothetical antibody process using a QbD framework.
Testing Requirements
The studies needed to show comparability and effectiveness will vary based on the extent of the differences between two resins, as well as the options being evaluated (such as changes within a particular method, altering the order of steps, or switching to a different method). The authors give some examples for a Protein A resin:
In general, if the alternative resin is similar to the original, the operating conditions will also be similar, although some adjustments may be necessary. However, if a change in modalities (i.e., HIC vs IEX vs affinity) or formats (packed bed vs. membrane adsorber) is being considered, more characterization studies are required to demonstrate comparability. A partial list of product quality attributes which may need to be assessed as part of a change is outlined.
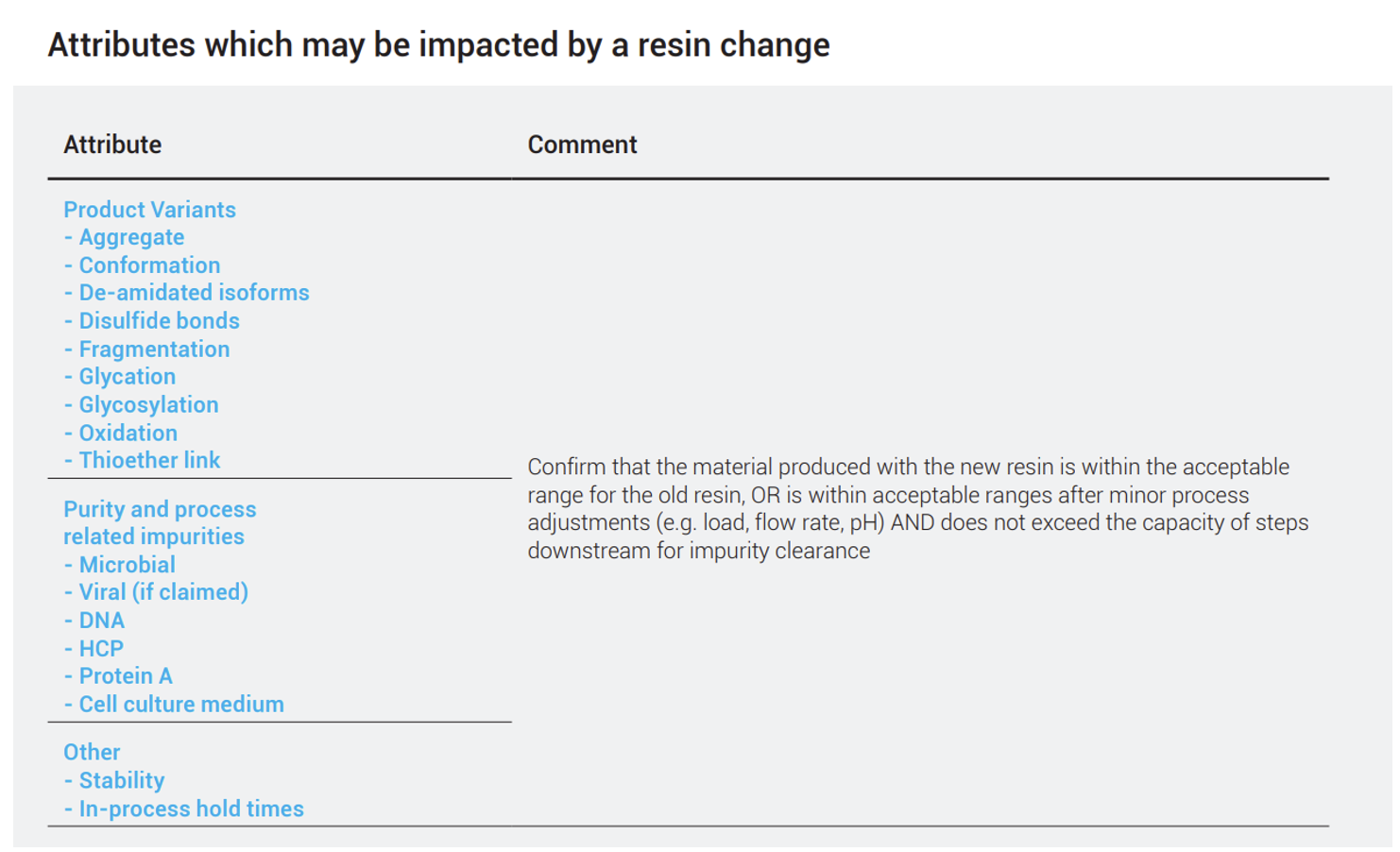
To wrap off this first half of the white paper, the authors go through several real-world examples from companies including Genentech, Amgen, and Biogen for implementing post approval changes to their drug product manufacturing processes.
Materials & Supplier Considerations
When considering a change in resin material, it is important to assess not only the materials specification, but also the supplier’s themselves. Changing the resin material will likely involve changing the supplier, triggering additional activities such as audits and material characterization. The risks associated with the change include supplier-related, material-related, and process impact.
Supplier
The authors emphasize several key areas of focus when evaluating a new supplier, which includes their quality systems, technical expertise, and business practices/health. Auditing a new supplier is essential to ensure there is an appropriate quality system in place, which should encompass change management and notification, management of non-conformances (NCs), and corrective action preventive action (CAPA) procedures and extends to their management of sub-suppliers.
From a technical side, suppliers should have a detailed understanding of their own processes, how their products are used, be able to support investigations, and be willing to share details regarding their manufacturing including the critical materials and controls used, which is important if change management/impact assessment is required. An emerging capability is whether a supplier can support data exchange/management in the digital Pharma 4.0 era to provide enhanced data analytics that can support rapid decision making. While not a requirement, this can demonstrate that a supplier is forward-thinking and committed to implementing modern technologies and processes.
Finally, while a supplier’s business health is not a quality issue, it does influence supply continuity. Due diligence is recommended to assess business health and stability, capability to meet capacity requirements, disaster recovery plans, intellectual property/freedom to ensure a reliable and sustainable long-term partnership.
Material
From a materials perspective, the authors recommend documenting the process of selecting and characterizing materials intended for a drug or biological product so decisions can be reviewed later as part of product lifecycle management. The authors note that a supplier may not provide all the available data for a given material, so it may be necessary to request additional information. For instance, suppliers seldom provide information on pore size distribution, but this is very indicative of resin performance and valuable information during assessment.
The inherent chemical properties of the reagents and processes used to produce a specific material can introduce potential risks. A good understanding of the material toxicity so companies can take appropriate steps to protect the patient through detection and/or removal methods. This also enables informed assessments of any changes communicated as part of a change management protocol. In the case of resins, the material risks fall into three categories:
- Materials/solvents used in the manufacturing process
- Solvents used as preservatives
- Leachables from the base matrix (resin) or the ligand
Resins are complex and can consist of multiple components, as is the case in pre-packed columns. It may not always be practical to perform testing on every component, so the user must rely heavily on the supplier’s quality systems to manage the quality of their incoming materials. Regulatory Support Files are usually provided by resin suppliers, containing information on the raw materials and their sources (as potential leachables), solvents (along with their clearance through washing procedures), and leachables studies. This emphasizes the importance of identifying a suitable resin supplier who has all the necessary quality systems and expertise to support change management activities.
As has been emphasized throughout, it is essential to follow established regulatory guidelines when considering changes to the manufacturing process or materials to ensure that they do not adversely affect the product’s safety, efficacy, or quality. Failure to comply with regulatory guidelines can result in costly delays or regulatory action, potentially leading to negative impacts on patient safety and product quality. The guidelines provided in the white paper serve as a general reference for developing a protocol, but the authors emphasize it is important for license holders to communicate with the appropriate regulatory authorities to engage in a dialogue throughout the process regarding their specific situation to ensure success and head off any unforeseen setbacks.
