
Flexible Downstream Platform Facilitates Adaptation to Scale
In biomanufacturing, upstream titers are increasing as new cell lines and technologies are being developed. This puts pressure on downstream processes, especially the capture step. To help biomanufacturers better adjust downstream processes to scale, GE Healthcare Life Sciences now launches its prepacked ReadyToProcess™ columns in two additional bed heights.
Column consistency facilitates process planning
The format of the ReadyToProcess columns is standardized, and the columns are manufactured using verified and validated methods optimized for each included chromatography resin. This enables a consistent column performance with minimal variation between column lots, simplifying planning of the downstream process even with a significant variation in upstream titers.
Adjusting mass throughput
At a fixed column diameter and by keeping residence time constant, the three bed heights have shown to provide comparable results for single cycles. However, by increasing the bed height, more product can be processed in a single cycle (Fig 1).
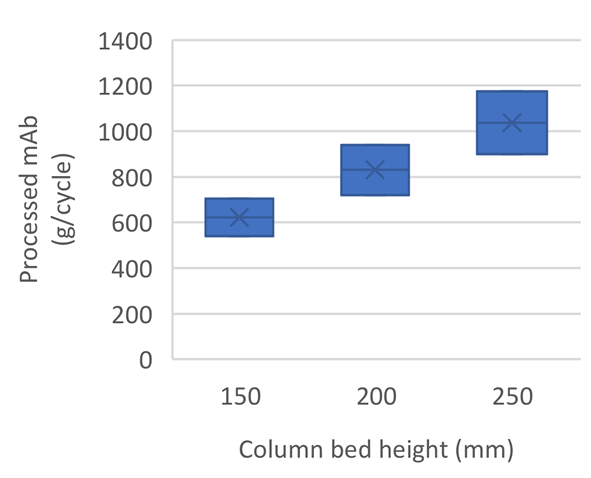
Adjusting cycle number
The number of cycles can also be varied for even greater flexibility. At a fixed column diameter, increasing the bed height allows for processing of the same amount of product in fewer cycles, ultimately reducing the process time (Fig 2).
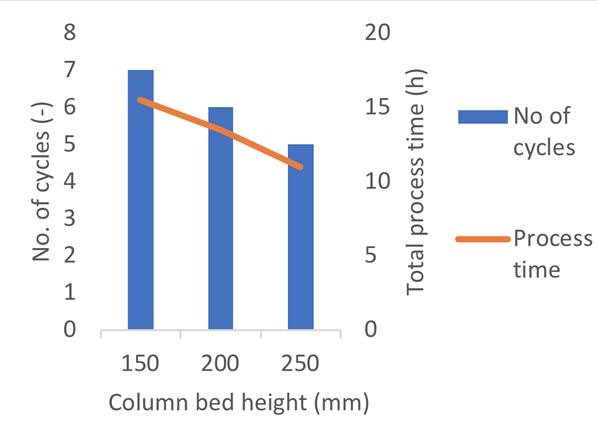
Learn more
As shown, depending on the selected bed height, ReadyToProcess MabSelect SuRe LX columns with 359 mm inner diameter can be used in capture of 540 g mAb in one cycle (150 mm bed height, mAb load of 36 g/L) to 5000 g mAb in five cycles (250 mm bed height, mAb load of 47 g/L). The presented examples demonstrate that a downstream platform comprising one column diameter, but including columns of three different bed heights, can be used with minimal adjustments to process varying upstream titers.
Learn more about ReadyToProcess columns
