
Future-proof Bioprocesses: Flexible Single-use Technology that Adapts to an Evolving Industry
The one constant in biopharmaceutical manufacturing is change. There is an ever-present need to adapt to new therapeutic modalities, more cost-effective approaches, higher product demands, and now a worldwide pandemic. Thus, the best way to ensure efficient biomanufacturing is to future-proof bioprocesses with flexible systems that enable companies to respond to changing priorities, new opportunities, and increasing demands.
Processes frequently change as a result of new enabling technologies. The advent of single-use systems, perfusion culture, as well as better analytical tools and sensors are just a sampling of the technologies that have changed bioprocessing over the past decade; however, implementing these improvements is not straightforward. Bioprocesses are not always built with the kind of flexibility that allows them to quickly adapt to improvements.
Another area that requires flexibility and adaptation is material supply. The industry is currently experiencing significant equipment and raw material shortages, largely due to the impact of the COVID-19 pandemic. However, some materials are prone to shortage, and they were difficult to source even before the pandemic. While we do not anticipate pandemics of this magnitude on a regular basis, it is important to be able to adapt and respond to adjustments in supply.
Changes in product manufacturing demand also require adjustment to process and scale. As a result, biopharmaceutical companies are seeking manufacturing that is easily scalable. Single-use systems and scale-out approaches to manufacturing have enabled this movement. There is no doubt that these strategies will continue to be in high demand as we move toward new therapeutics that require smaller volumes, such as cell and gene therapies.
Balancing Act: The Biotech Industry Demands Flexible Operations
While the needs of scientific research versus current good manufacturing practice (cGMP) are sometimes different, the nature of the biotech industry is such that labs must be flexible enough to move between the two realms. Product starts and is purified in the lab before it makes its way to process development, clinical trials, and cGMP production. But the strict regulations of cGMP production can inform how labs are designed, particularly in terms of automation and process design, in order to ease process transfer. Whether a research lab or a small start-up focusing on early-phase production, labs designed with scalable instruments and robust digital control platforms could set themselves up to secure future investment and/or scale-up opportunities.
The biotech world is an ever-evolving industry, with new technologies coming to market at rapid rates that may not only change a company’s equipment needs, but also its process needs. The adoption of single-use technologies, for example, has boomed in the past decade as the industry moves toward smaller-scale bioprocesses. Spearheaded by recent developments in medical research, and in personalized medicine and gene therapy in particular, biotech facilities are shifting from high-volume productions to multi-batch production of smaller-scale product. The result: where stainless steel was conducive to large-volume production, the time spent cleaning and sanitizing in between batches — paired with increasingly strict regulatory guidelines — has made disposable technologies critical from a commercial perspective (1).
Being able to reconfigure processes and facilities for changing needs is essential in this industry. Where oncology dominated biotech manufacturing in 2019, the global pandemic shifted the manufacturing focus towards infectious disease, disrupting more than 1,000 clinical trials worldwide (2). What’s more, the messenger RNA-based (mRNA) vaccines produced by Pfizer and Moderna in response to COVID-19 have shown us that vaccine types require different technologies and processes to manufacture (2). Some more traditional vaccine facilities may not be easily repurposed for mRNA vaccine production, which demands further upstream processing, purification steps, and strict storage requirements (3).

Responding to the times requires flexible technologies and systems as well as flexible partnerships. Issues like capacity and inventory control have led some companies to reconsider how much manufacturing is being produced in-house or outsourced. The expedient vaccine response to the pandemic was in part thanks to successful partnerships between sponsor companies and contract development manufacturing organizations (CDMOs) — which allowed swift transitions from R&D and clinical production to large-scale manufacturing (2). But while CDMOs will continue to expand, Joseph Rininger of Latham BioPharm Group notes that many cell and gene therapy (CGT) companies are increasingly investing in their own production networks (4).
“[It] allows developers to have more control over their initial timelines for getting into the clinic, which then can lead to the next series of money and advancement of their product pipeline and company.” (4)
Throughout 2021, biotech companies have been pressured to balance non-pandemic and COVID-19 therapies. Some CGT companies are finding the balance through hybrid outsourcing formats in which agreements with CDMOs secure capacity for later-stage projects, while R&D and early-phase production is kept in-house. Such collaborations are an important part of a company’s flexibility strategy, particularly for startup CGTs that need to be careful about where they allocate funds. For those investing in facilities, there are several design considerations to keep in mind to future-proof their bioprocess.
Flexible Technology: Challenges & Solutions
Collaboration
A key tool for ensuring that a process is flexible and adaptable is through collaboration. There needs to be good communication between the biopharmaceutical companies, suppliers, and regulators to ensure that the industry can be nimble in responding to internal and external changes. A good collaboration can also create a process that exactly fits the purpose for which it was designed. For instance, in cell and gene therapy manufacturing, there has been a shortage of process solutions designed specifically for those applications at the appropriate scale. In response, developers have had to patch together technologies designed for traditional protein biologics to create solutions. Since these technologies were originally designed for a very different type of workflow, they may not fit the needs of cell and gene therapy well — and this can lead to an inefficient and costly workflow that is not future-proof.
Fully Customizable Fit for Purpose
The biotech industry requires flexible operations to move swiftly between R&D and GMP, evolve with innovating technology, and respond to shifting industry needs like the unprecedented COVID-19 pandemic response. But flexible operations depend on flexible processes, which are predetermined by lab and biomanufacturing hardware and software technology.
One of the major challenges to flexibility in the lab is technological incompatibility — whether for upstream or downstream processes. While customizability of a platform process is the primary goal, the reality is that customizing an existing platform has limitations.
Market-leading suppliers offer a range of competitive single-use systems with some flexibility within their platform of products. However, full flexibility of these products is often limited: they are flexible in that they are single-use, facilitating quick and sterile batch changes between production runs, but the instruments themselves are not always adaptive to unique process needs and typically do not integrate products and/or components outside of brand. There is a need in the industry for more flexible solutions that can be tailored to specific bioprocessing needs, as well as solutions that can adapt and evolve as process requirements change. Agilitech is one company that is focused on delivering single-use technologies for upstream and downstream bioprocessing that meet the increasing demand for flexible solutions.

Agilitech offers a different approach to flexibility by providing systems that can be tailored to specific needs and integrate fit-for-purpose workflows. For example, Agilitech’s product design team worked with a customer to tailor its standard single-use TFF system to meet very specific requirements, including adapting the skid to accommodate different filter sizes and flow rates. The company has also developed a unique TFF system that can be used at small scale and then adapted for larger-scale production with minimal modification. This type of flexible design not only streamlines and accelerates the scale-up process, but eliminates the need to purchase new skids at each scale-up stage.
The engineers at Agilitech are sensitive to the fact that scale is particularly important to the gene therapy sector — not only in terms of manufacturing and production, but also in terms of optimizing a specific bioprocess. Dennis Hodgson, Agilitech Product Manager, comments:
“The market is somewhat limited in terms of chromatography systems optimized for viral-based therapies, which can deliver millions of particles per millivirus in a very small volume. Where other single-use chromatography systems have flow rates starting at 30mL/min, Agilitech offers 1mL/min flow rates on a single-use system. With a dynamic flow range starting at 0-500 mL/min up to 20 LPM, Agilitech can optimize the skid for a specific process without over-sizing production. It’s a true fit-for-purpose solution.”
Agilitech works with customers to co-design process-based solutions that meet their exact requirements. By listening to specific customer needs and then designing a 3D model of a skid in collaboration with the customer, Agilitech adapts
its standard system design to provide an exact fit-for-purpose solution. This front-end planning and collaborative design process is no more costly than other available products, while providing significant benefits to the overall workflow.
With this unique approach, Agilitech creates solutions that truly fit each unique process. In biomanufacturing one size rarely fits all — thus, tailored solutions that involve the customer in the workflow design and implementation is critical.
Brand-agnostic Systems
Agilitech products can also be reconfigured to use filters, sensors, and other components from virtually any manufacturer brand. The use of brand-agnostic components adds another level of flexibility. For example, customers can use their preferred filter brand with their preferred sensors along with a custom-configured flow path. This enables them to fully customize their bioprocess while minimizing the equipment operation/maintenance learning curve and their spare parts inventory. This unparalleled flexibility means that users become co-designers of their equipment and they can continue to evolve their instruments according to changing needs and evolving technology.
ln other words, Agilitech’s brand-agnostic technology provides users the freedom to work with the companies and products that best meet a workflow need. And as we’ve seen in 2021, the ability to partner and collaborate with others has never been more important. Process equipment with the flexibility to use components from different vendors and brands can also help to mitigate supply chain issues such as those experienced during the COVID-19 pandemic. For Agilitech, collaboration is not only about harnessing best practice, but also about innovating at an affordable cost and with reduced risk.

Paradigm Shift
Change can be difficult within the industry, and there is sometimes a perception that the cost and lost time associated with new workflows, qualification of new components, and technological investment may not be worth the effort. However, we have seen time and time again that employing innovative technologies as well as the ability to evolve and do so quickly is key for speed to market, meeting quality attributes, and cost-effective manufacturing.
As an increasingly complex industry in terms of data output, a major pain point for biotech companies is balancing their need to improve processes through automation and integration, while also investing in human resources to properly analyze data and make sure the company stays compliant to industry audit regulations. A solution to abating fears around analytical process change is through expert consultancy. But finding the right team for the job isn’t always easy, as Steve Martin, Vice President and Head of Biopharm Molecular Discovery at GSK noted at the 2019 Lab of the Future Congress:
“Data is becoming much more important, yet scientists with data analysis skills and biological or chemical backgrounds are very rare. Getting these two groups to talk to each other in the same language is a problem.” (5)
Software is one of the greatest challenges to a company’s flexibility and a major barrier to the industry’s progress. Bryn Roberts, Senior Vice President and Global Head of Operations at Roche told Lab of the Future Congress members that,
“What is slowing digitalization is the lack of a common operating system…an orchestration backbone.” (5)
Current lab systems often produce data output in non-user-friendly formats that are burdening scientists with data conversion — or making them increasingly reliant on data analysts before they can do meaningful analysis.
Agilitech is a company that leverages a strong engineering background to approach problem solving from a process optimization perspective — looking at the whole as well as the parts. With broad automation experience on Delta V®, Rockwell Automation®, and other leading platforms, Agilitech offers both problem-solving and integration services. The goal is to work with the customer to understand their pain points and find a solution using the existing hardware and software components that they have.
While automation software is designed to streamline processes, few have the robustness to support a complete lab bioprocess and its eventual scale-up at cGMP manufacturing levels. For this reason, Agilitech uses Delta V®, Rockwell Automation®, and other leading automation/control platforms that harness industrial power and provide an adaptable interface. This helps to ensure ease of process transfer for a growing company and offers a flexible platform for customizability. Users can also make system modifications on their own if they have the resources to do so. Where others often play catch-up to adapt their system software to emerging technology innovations, Agilitech control systems are designed for change.
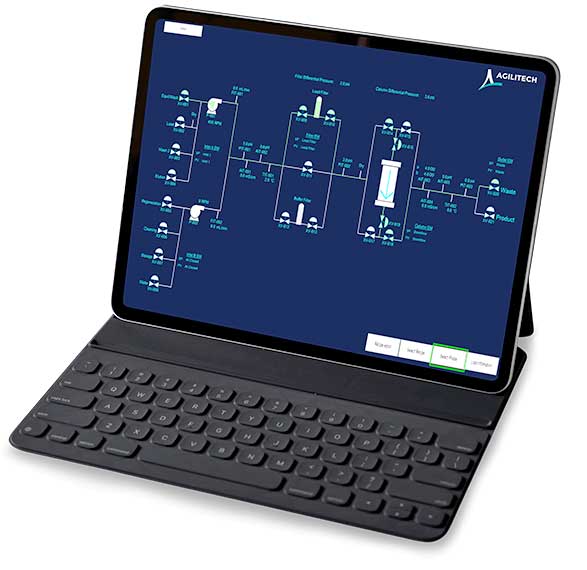
Agilitech’s flexible approach to automation and bioprocess control also enables seamless integration of equipment from different manufacturers — and helps to eliminate the islands of automation that are often found in biotech laboratories.
Future-proofing Means Designing for Change
The biotech industry is ever-evolving and demands that we design technology with flexibility in mind. Full flexibility requires equipment that is easily tailored to specific needs, reconfigurable, and able to evolve with the workflow to incorporate technological innovation and address changes in supply or demand. It also means having instruments and operating systems that are brand-agnostic, which enables scientists and lab technicians to create fully customizable processes and operations that fit their production needs. This will allow scientists to not only design their “dream process,” but also frees them to forge partnerships with a variety of vendors and suppliers to optimize their engineering process (both efficiently and cost-effectively). It is important for today’s providers of single-use and other bioprocessing technologies and services to focus on understanding and accommodating unique customer needs in order to provide solutions that are truly fit-for-purpose with the flexibility required to adapt to future stresses or demands on the process.
To download a copy of this article, please visit –
Future-proof Bioprocesses: Flexible Single-use Technology that Adapts to an Evolving Industry
For more information on Agilitech and the company’s single-use technologies mentioned in this article,
visit: https://agilitech.bio
Agilitech is a pioneering partner to the biotech industry. The company helps to drive progress by designing and implementing state-of-the-art equipment and bioprocessing systems for biotech research labs through to full-scale production, along with game-changing bioprocess engineering and automation services.
In a fast-moving industry that is constantly evolving, Agilitech has the flexibility and experience to tailor its offerings to the specific requirements of each and every customer. A transformative product design and engineering process is what makes Agilitech single-use bioprocessing technologies unique and ensures that the company’s solutions meet the exact needs of each and every customer. Through front-end planning and collaborative product design with end users, Agilitech delivers truly fit-for-purpose solutions to real problems.
DeltaV is a trademark of Emerson Electric Co. Rockwell Automation is a registered trademark of Rockwell Automation, Inc.
Footnotes
-
1. High Purity New England. “Biotech Single-Use: What's Driving The Growth of Single Use?” High Purity New England, 22 Sept. 2020, hp-ne.com/biotech-singleuse-whats-driving-growth-singl e-use/.
-
2. Haigney, Susan. “Supporting the Industry During a Pandemic.” BioPharm International, 1 Jan. 2021, www.biopharminternational.com/view/supporting-the-industry-during-a-pandemic.
-
3. Schlake, Thomas, et al. “Developing MRNA-Vaccine Technologies.” RNA Biology, Landes Bioscience, 1 Nov. 2012, www.ncbi.nlm.nih.gov/pmc/articles/PMC3597572/.
-
4. “In-House Manufacturing: Cell & Gene Therapies.” BioProcess International, 6 Aug. 2020, bioprocessintl.com/bpi-theater/bpi-theater-bio-2020/will-in-house-manufacturing-capabilities-give-cell-and-gene-therapy-developers-competitive-advantages/.
-
5. Pearson, Sue. “Biopharma's Lab of the Future Can't Wait.” GEN: Genetic Engineering & Biotechnology News, 3 Jan. 2020, www.genengnews.com/insights/biopharmas-lab-of-the-future-cant-wait/.
-
6. Chow, Denise. “What Is MRNA? How Pfizer and Moderna Tapped New Tech to Make Coronavirus Vaccines.” NBCNews.com, NBCUniversal News Group, 17 Nov. 2020, 23:08, www.nbcnews.com/science/science-news/what-mrna-how-pfizer-moderna-tapped-new-tech-make-coronavirusn124805\

