
Impact of Continuous Chromatography Mode on Protein A Resin Lifetime
Introduction
Traditionally, Protein A chromatography is performed in batch mode using a single, packed column. In batch operations, antibody-containing samples are loaded onto the column at levels well below the total capacity of the resin to prevent sample breakthrough and subsequent product loss. However in recent years, continuous chromatography has emerged as an alternative to batch operations to improve productivity or increase resin capacity utilization of chromatography purification processes. Continuous chromatography by periodic counter-current chromatography (PCC) has been demonstrated to increase utilization of the chromatography resin capacity (1). As illustrated in Figure 1, PCC enables loading to levels significantly higher than in batch processing (2).
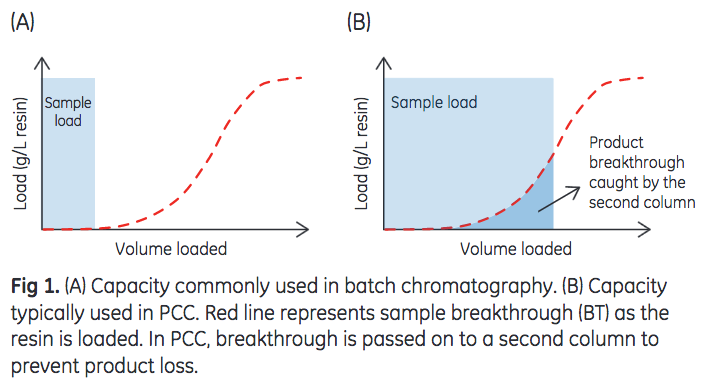
However, as columns are loaded beyond the point of breakthrough, this method of operation could potentially reduce resin lifetime caused by, for example, resin fouling. Given that resin lifetime is an important factor in determining overall resin productivity and cost, it would be desirable to achieve the same resin lifetime regardless of the mode of operation, that is, batch or continuous. The purpose of this study is two-fold: (i) to determine if the large sample loads employed in continuous chromatography affect resin lifetime, and (ii) to determine if a resin that is designed for PCC performs similarly to a conventional high-capacity resin in a continuous capture application.
Materials and methods
MabSelect SuRe LX™
MabSelect SuRe LX is developed for high-titer mAb capture at process scale. Optimized cross-linking of the agarose base matrix allows for, at minimum, five-fold higher fluid velocities as compared with conventionally cross-linked agarose. The Protein A-derived ligand exhibits high alkali-stability, enabling the use of rigorous and cost-effective cleaning-in-place (CIP) and sanitization protocols based on 0.1 to 0.5 M NaOH. The high protease tolerance of the resin ensures low ligand leakage. At extended residence times, MabSelect SuRe LX exhibits an enhanced dynamic binding capacity. In a previous study in batch mode, it was demonstrated that MabSelect SuRe LX resin maintains more than 95% of its initial dynamic binding capacity (DBC) after 100 cycles, using cell feed containing 0.8 g mAb/L as sample (3). MabSelect SuRe LX was also shown to provide a high and stable mAb recovery (> 97%), with leached Protein A and host cell protein (HCP) maintained at consistently low levels.
MabSelect SuRe pcc™
MabSelect SuRe pcc offers exceptional capacity at short residence time, making it well-suited for applications requiring fast mass transfer, such as mAb capture in a continuous process. The resin uses the same alkali-stable Protein A-derived ligand as MabSelect SuRe LX, coupled at the same ligand concentration, although on a smaller high-flow agarose bead. The base matrix combines a porosity optimized for mAbs with a rigidity that delivers good pressure/flow properties, allowing high flow rates and thereby increasing productivity of continuous capture steps. The small bead size also creates possibilities for high-resolution purification, for example, for purification of bispecific antibodies.
Sample preparation
Chinese hamster ovary (CHO) cell culture, containing 4.0 g mAb/L, was harvested by centrifugation followed by filtration using an ULTA™ Pure HC 0.6/0.2 μm filter. Capture of mAb Four 1 mL Tricorn™ 5/50 columns (5 cm bed height , 0.5 cm i.d.) were packed with MabSelect SuRe LX and MabSelect SuRe pcc, two columns for each resin. The columns were installed on an ÄKTA™ pcc 75 chromatography system with the capacity of running four columns, where the feed is constantly applied to two columns in the loading zone and the columns are switched between the loading and non-loading steps such as wash and elution (Fig 2). For this experiment , the 2 mm UV cells of the ÄKTA pcc 75 system were exchanged for 0.4 mm UV cells to enable undiluted sample feed to be loaded directly onto the column.
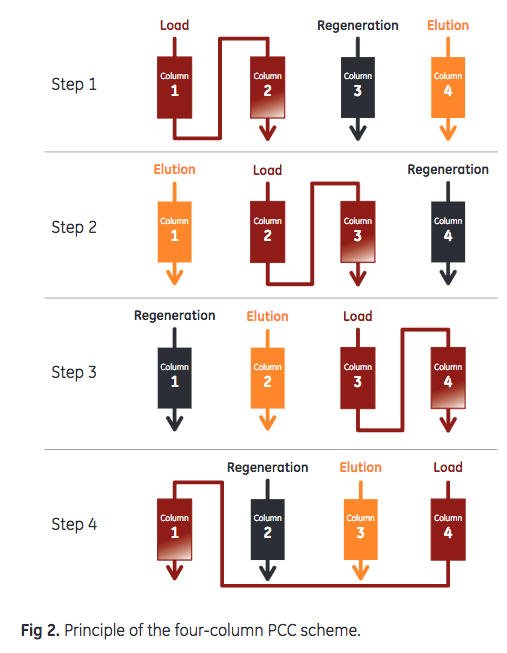
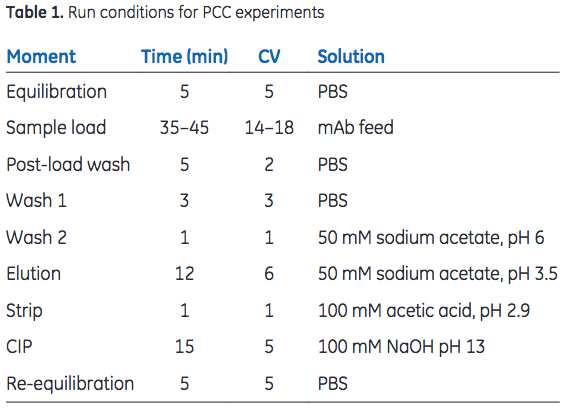
Sample was loaded (residence time = 2.5 min) onto the series-connected load columns to 35% breakthrough. The columns were washed with 2 + 3 column volumes (CV) of phosphate- buffered saline (PBS), followed by 1 CV of 50 mM sodium acetate, pH 6, before bound mAbs were eluted with 6 CV of 50 mM sodium acetate, pH 3.5. After a strip step, using 100 mM acetic acid at pH 2.9, CIP was performed with 0.1 M NaOH (15 min contact time). Run conditions are listed in Table 1. DBC was monitored through the sample load volume at 35% breakthrough and area under the curve (AUC) of the elution peak. A constant sample load volume indicates a consistent DBC throughout the study. The AUC corresponds to mAb recovery and gives an indication of the integrity of the column.
Determination of HCP and Protein A content
HCP content in the elution fractions and product pools were analyzed using commercially available anti-CHO HCP antibodies (Cygnus Technologies Inc.) and Gyrolab™ workstation (Gyros AB). Protein A content was determined using a commercially available ELISA kit (Repligen Corp.).
Results
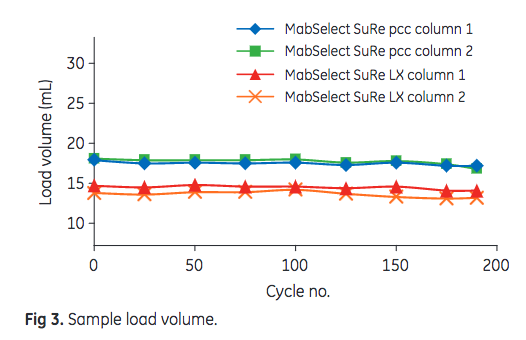
In this study, 175 PCC cycles (i.e., complete purification protocol that one column is subjected to) were performed. Leached Protein A, HCP, and mAb content in the eluate were used as output parameters, indicating resin performance. The dynamic control functionality of ÄKTA pcc 75 is used to adjust for variations in resin binding capacity or changes in feed composition. The system uses the dynamic control functionality to switch columns when the preset breakthrough level is reached. To detect changes in DBC, the sample load volume can be used. As shown in Figure 3, the sample load volume after 175 cycles was at least 95% of the initial volume for all four columns. MabSelect SuRe pcc exhibited an approximately 25% higher DBC than MabSelect SuRe LX.
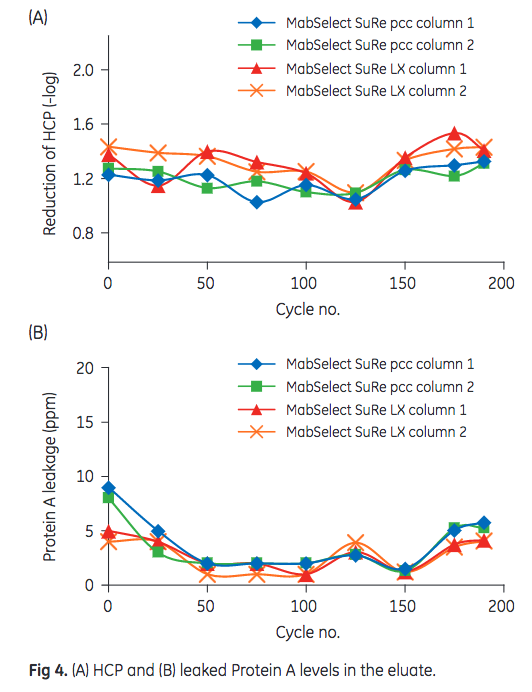
HCP and leached Protein A content in the eluate are depicted in Figure 4. As shown, levels remained constant over the course of the 175 cycles and no significant difference between the performance of MabSelect SuRe LX and MabSelect SuRe pcc could be observed.
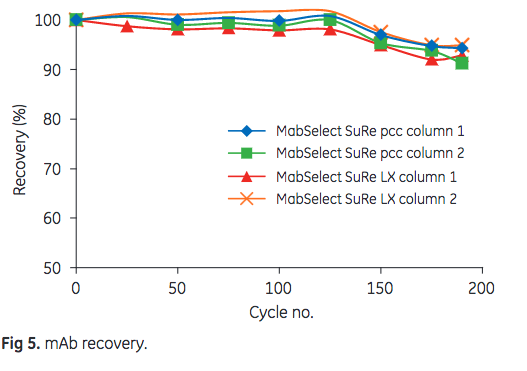
As can be seen in Figure 5, a slight reduction in mAb recovery was observed after cycle 125. After 175 cycles, however, the final mAb recovery was still more than 90%.
Conclusions
The presented data demonstrates that PCC does not compromise the performance of MabSelect SuRe LX or MabSelect SuRe pcc over the performed 175 cycles, supporting the use of this technique to improve resin capacity utilization against batch processing. While the performance indicators (HCP, leached Protein A, and mAb content in eluate) were comparable between the resins, MabSelect SuRe pcc demonstrated an approximately 25% higher mAb binding capacity compared with MabSelect SuRe LX under the conditions used. The results confirm that the use of a resin designed for PCC can help improve economy of continuous processes even further.
For more information, please visit www.gelifesciences.com/bioprocess
Footnotes
-
1. Application note: Continuous chromatography in downstream processing of a monoclonal antibody. GE Healthcare, 29170800, Edition AA (2015).
-
2. Application note: The use of dynamic control in periodic counter-current chromatography. GE Healthcare, 29169950, Edition AA (2016).
-
3. Application note: Lifetime performance study of MabSelect SuRe LX during repeated cleaning-in-place. GE Healthcare, 28987296, Edition AA (2011).
