
Intensify Process Development Steps and Simplify Transfer to GMP Environment with New ÄKTA Pilot 600 Chromatography System
The bioprocess industry is leaving the blockbuster era. The strive to shorten time to market is constant, which puts pressure on the entire drug development process from research to manufacturing. With more drugs in pipeline, biomanufacturers need flexibility, efficient changeovers, and accelerated scale-up and transfer to the next development phase.
Tools that help intensify process development steps and simplify the transfer to GMP environment can help get you there. The new ÄKTA™ pilot 600 chromatography system from GE Healthcare Life Sciences is a great example. It is a compact, bench-top system with a wide flow and pressure range. It suits both production of technical batches and scale-up studies as well as small-scale production of GMP-grade material.
Adapt your system to meet your process needs over time
Process development is all about optimization. To maximize outcomes, the ÄKTA pilot 600 can easily be adjusted as requirements change over time. This is thanks to a modular design. For example, as you are finetuning your process in late stages of process development, more outlets are often required. On the other hand, as you move up in scale and aim at automating the process, you often want more inlets and sensors.
Module assembly is easy using a supplied tool and quick activation in UNICORN software. Once up and running, the interactive process picture allows changes to be made in real-time and deviations are quickly identified.
The system is available in two versions, ÄKTA pilot 600S and ÄKTA pilot 600R. The 600S model is suitable for non-GMP environments while 600R fits GMP environments. The set-up of both versions can be adjusted thanks to the modular design.
Easy and robust packing, the foundation of a reliable chromatography process

ÄKTA pilot 600 combines smart hardware design with dedicated software features. As an example, the system can help you achieve well-consolidated chromatography beds quickly. The simple and interactive graphical workflows for Intelligent packing in UNICORN aid in both column packing and testing packing procedures.
Packing progress can easily be monitored in the process picture and with the time to bed clearly visible. The integrated HETP workflow allows for one-click updates of column statistics. From a hardware perspective, the system has been equipped with two column positions and a dedicated AxiChrom packing port. This means that a second column can be connected without disconnecting the first one.
Minimize handling and changeover time

Making everyday workflows easy and simplifying change over procedures will speed up each project. ÄKTA pilot 600 is designed for sanitary environments. For example, the system chassis is easy to wipe down and have minimal areas where dust and liquid can get trapped.
In addition, novel SNAP-connectors remove the need for O-rings or welding, which might otherwise present a sanitary weakness. The system modules have been designed to have a clear distinction between wetted and functional parts. Finally, CIP procedures can be simplified by removing modules not required for the next campaign.
Reliably scale-up your process and simply transfer to GMP environments
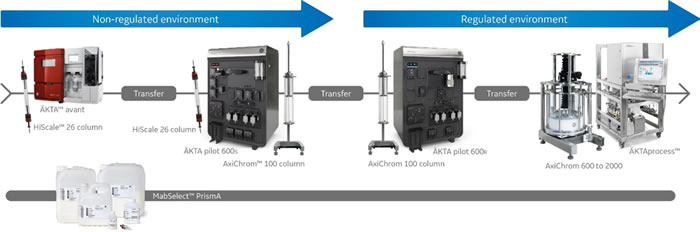
The wide flow rate and pressure range of ÄKTA pilot 600 enables more than 40-fold scaling for columns within a 26 to 200 mm column i.d. range. This wide range makes it an excellent system to bridge the transition into GMP environments. It also allows you to match product yield to project need and save both time and money.
The system scalability was demonstrated in this multidimensional scale-up study. It shows a 20-fold scaling from a lab-scale chromatography system and column up to an ÄKTA pilot 600 system and an AxiChrom 100 column.
Want to know more? Explore the new ÄKTA pilot 600 in 3D or check out the chromatography system details.
