
New Technology Solutions for Lentiviral Vector Clarification
The growth of the cell and gene therapy sector, and the growing number of gene and gene-modified cell therapies entering the market has translated into an increased global demand for lentiviral vectors (LVs) as a gene delivery tool. There are currently two FDA approved LV-based cell and gene therapy products—Kymriah® (Novartis) and ZYNTHEGLO® (bluebird bio), and according to the Alliance for Regenerative Medicine, an estimated 48% of the ongoing cell and gene therapy clinical trials use lentiviral vectors, with a significant number in late-stage evaluation1. The strong pipeline of LV-mediated gene and CAR-T cell therapy products headed towards market approval means the development of efficient LV vector purification methods to improve yield and recovery is needed to meet the growing demand while also driving down the cost of production2,3.
The critical first step in the downstream process is clarification of the viral bulk, which aims at removal of cell and cell debris and to reduce turbidity of the solution. Since each process step potentially reduces the quantity of infectious LVs, effective clarification helps minimize LV vector loss from the outset of the downstream process to maximize final product recovery4. In this publication, we will examine counterflow centrifugation (CFC) as a scalable technology solution to achieve high throughput and high yield clarification of infectious LVs.
Introduction
Lentiviral vectors are typically produced by transient transfection of HEK293T cells using 3 to 4 vector plasmids. These highly labile, enveloped particles bud from the producer cells and are released into the supernatant. Clarification is used to physically segregate the cells from the LV-containing cell culture medium. Because of the fragile nature of LV, short process times and a limited number of process steps are essential for maintaining the activity of the vector2,4,5. Additionally, known issues with adsorption to filtration membranes makes it difficult to achieve effective LV clarification with standard bioprocessing technologies. Inappropriate clarification processes can result in filter clogging, which reduces the actual pore size of the filter leading to LV vector retention and reduced product yields leading to oversized and expensive production batches4.
Centrifugation is a lab-scale method used for clarification; however, traditional centrifugation can increase the risk of batch contamination, introduce shear stress that can damage the membrane envelope, and is a technique that is not practically scalable for large volume processing. Counterflow centrifugation (CFC) is a gentle yet powerful technology that can be leveraged to effectively clarify lentiviral vector supernatant. The Gibco CTS Rotea Counterflow Centrifugation System and Sartorius Ksep400 counterflow centrifuges jointly provide automated workflow solutions for small- to large-scale lentiviral harvesting within closed systems to enhance viral recovery with low shear forces with the highest level of containment and product safety protection.
Counterflow Centrifugation Basics
Traditional centrifugation uses centrifugal forces to separate cells and cell debris based on size and density, with larger and denser particles pelleting at lower G forces. During CFC, the system suspends materials in a fluidized bed within a funnel-shaped chamber by exerting an additional and opposite constant “counterflow” force to the centrifugal forces that enables effective separation without pellet formation in the cone. The key concept is that cells and particles migrate within the chamber to different positions within the fluid based on their sedimentation rates until the two opposing forces upon them reach equilibrium. Larger/denser cells are captured in the fluidized bed, while smaller/less dense cells and particles, such as LV vector, pass through and are elutriated through the top of the CFC chamber (Figure 1).
For LV clarification, CFC effectively removes the virus producing cells with minimal cell disruption and enables collection of the viral vector-containing supernatant as it is elutriated from the chamber. Separating the cells intact from viral supernatant minimizes contamination from host cell DNA, protein and debris caused by cell disruption, resulting in higher viral purity in the LV supernatant from the start. The supernatant can then be further processed by filtration through a 0.45 µm low protein binding filter or other equivalent method to achieve a high yield clarified output with low turbidity which can then be subsequently processed with other downstream unit operations.
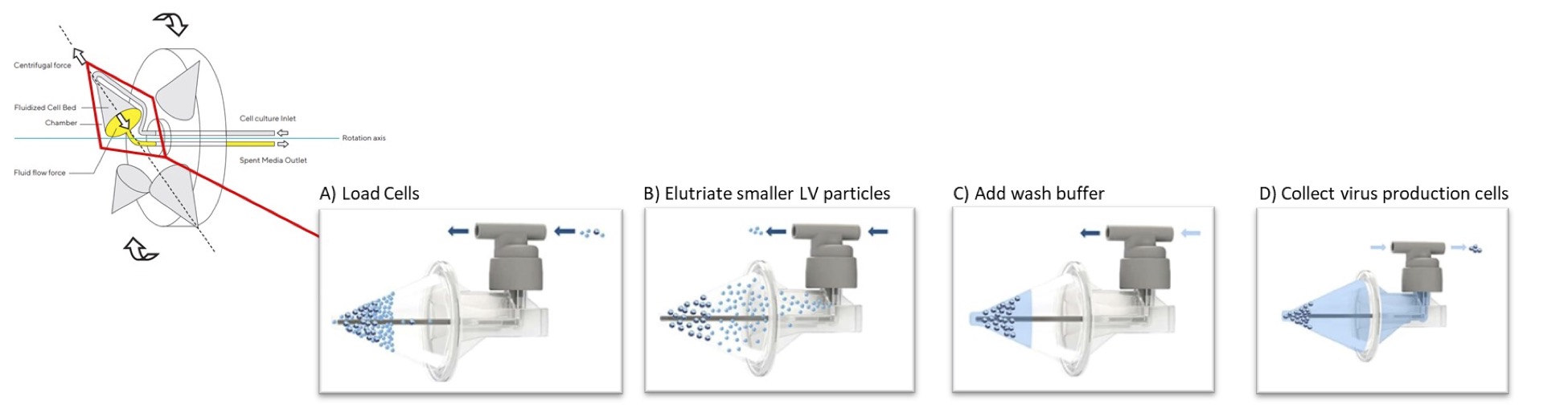
Benefits of Automated Closed CFC Systems for LV Workflows
Scalable, closed alternatives to traditional centrifugation can greatly benefit LV downstream processes to effectively clarify LV feed by removing producer cells and cell debris while maintaining high titers with minimal risk of contamination. Closed systems also minimize the need for biosafety cabinets, class C clean rooms and associated costs and allows for flexibility in facilities usage since multiple batches can be processed in a shared clean room. In addition, closed systems help the bioburden control strategy which should be in place in any GMP productions. The CTS Rotea System and the Sartorius Ksep 400 System are single-use centrifugation systems that provide CFC capabilities across a high range of production scales for different stages in the development and manufacturing.
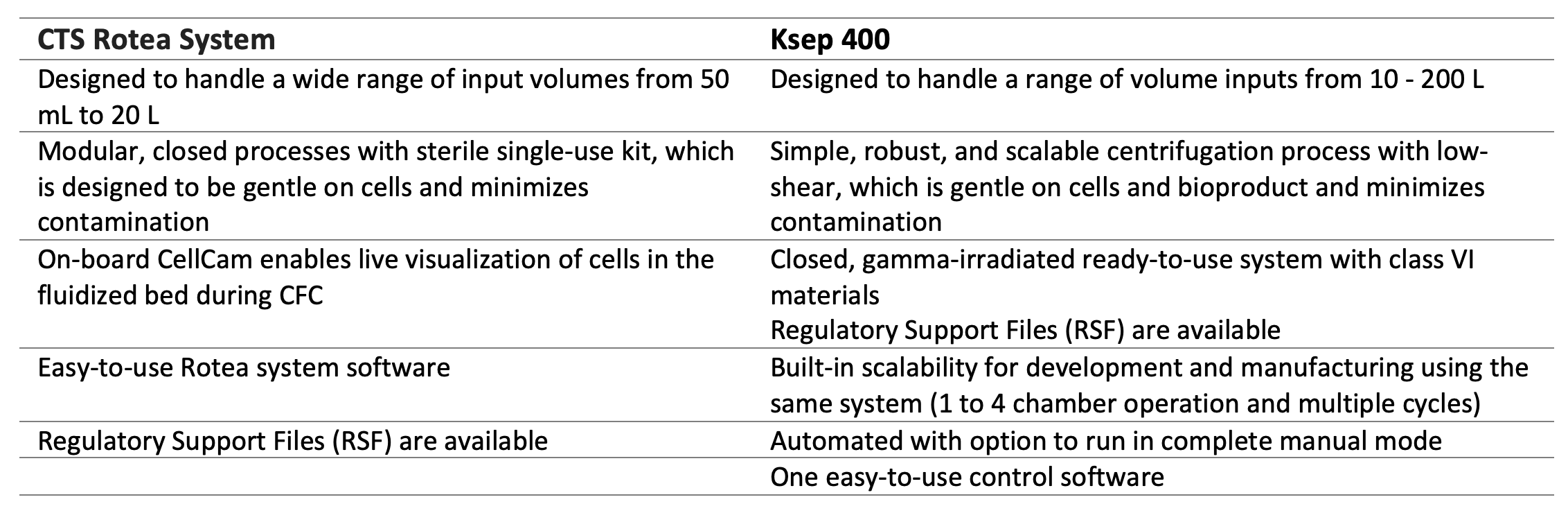
The CTS Rotea System can be easily implemented for small-scale LV clarification suitable for a wide range of input volumes to develop and refine process parameters that can be easily transferred to the larger Ksep 400 system to meet scale up requirements. Together the instruments can be used to scale LV clarification processes across a broad range of production volumes from small to large scale GMP batch volumes.
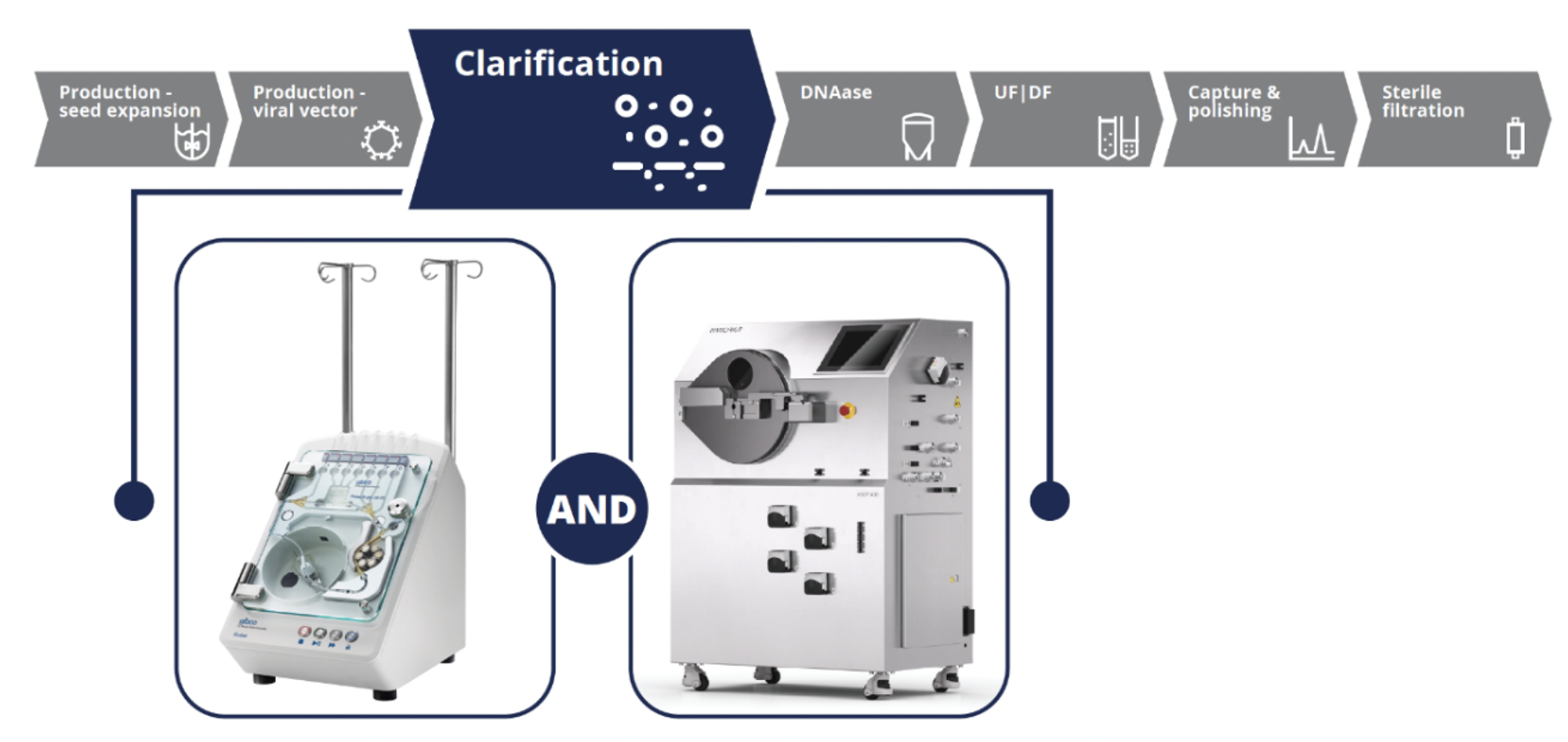
Performance Characteristics of the CTS Rotea System and Ksep 400 CFC Systems
To demonstrate the capabilities of both instruments for LV vector clarification, experiments were conducted using the CTS LV-MAX system following the workflow outlined in Figure 2.
In counterflow centrifugation, balancing the centrifugal and fluid flow force is critical to the successful operation of the instruments to effectively capture and separate VPCs from the viral supernatant with minimal cell breakthrough. Flow rate and centrifuge G force settings are largely based on the size and density of the cells as well as the density and viscosity of the cell culture media. While these settings can be approximated using modeling systems and empirical knowledge of other similar cell types, they should be optimized for each specific cell production system to achieve a clarified output. The ratio between these two forces can be used to adjust these speeds without impacting the systems’ ability to capture cells in a fluidized cell bed. If cells are being washed out from the exit port of the cone during initial cell bed establishment, decreasing the flow rate or increasing the G force would allow for more successful cell bed establishment (increasing the C/F ratio). These two values G force and flowrate are the critical parameters to consider during scale and optimization studies.
To determine the clarification performance, turbidity reduction, lentiviral particle titer, cell viability and removal were quantified for both instruments. The technical details for the experimental setup, instrument settings and analytical methods can be viewed in the Appendix.
-
Turbidity Reduction
After cell harvest from a typical LV bioreactor process, the turbidity of the unprocessed LV supernatant ranges between 300 and 550 Nephelometric Turbidity Units (NTUs) prior to clarification. The turbidity was measured before and after clarification to calculate the reduction in turbidity.
Clarification using both the CTS Rotea System or Ksep 400 Counterflow Centrifugation systems successfully reduced turbidity in the LV feed to <100 NTU and <30 NTU after filtration of the clarified harvest (Figure 3).
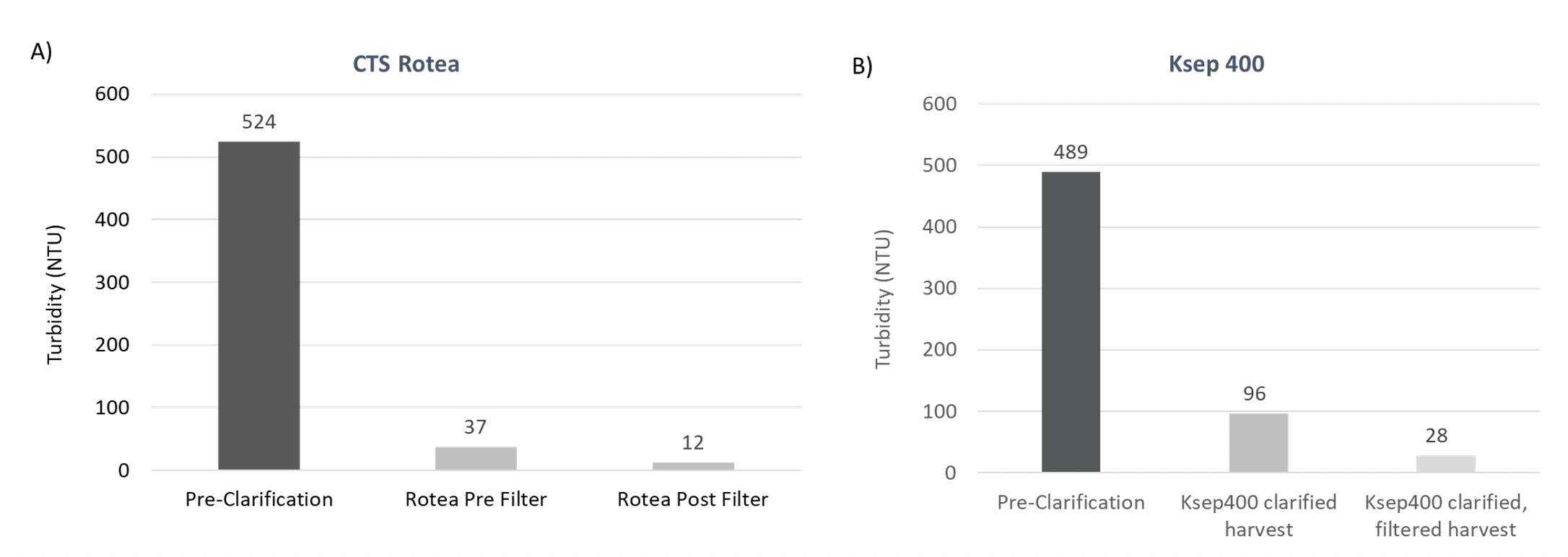
This data shows that the closed automated CFC systems can effectively clarify LV containing culture broth, significantly reducing turbidity by >80% of the input. The low turbidity readings also indicate that cells removed using CFC maintain high viability since cellular damage/lysis would contribute to higher turbidity readings and contributes to effective filtration post-clarification, minimizing the risk of filter clogging. Standard centrifugation where cells encounter high shear, can increase cell disruption, and introduce intracellular protein leading to extended processing times and add additional costs for consumables.
-
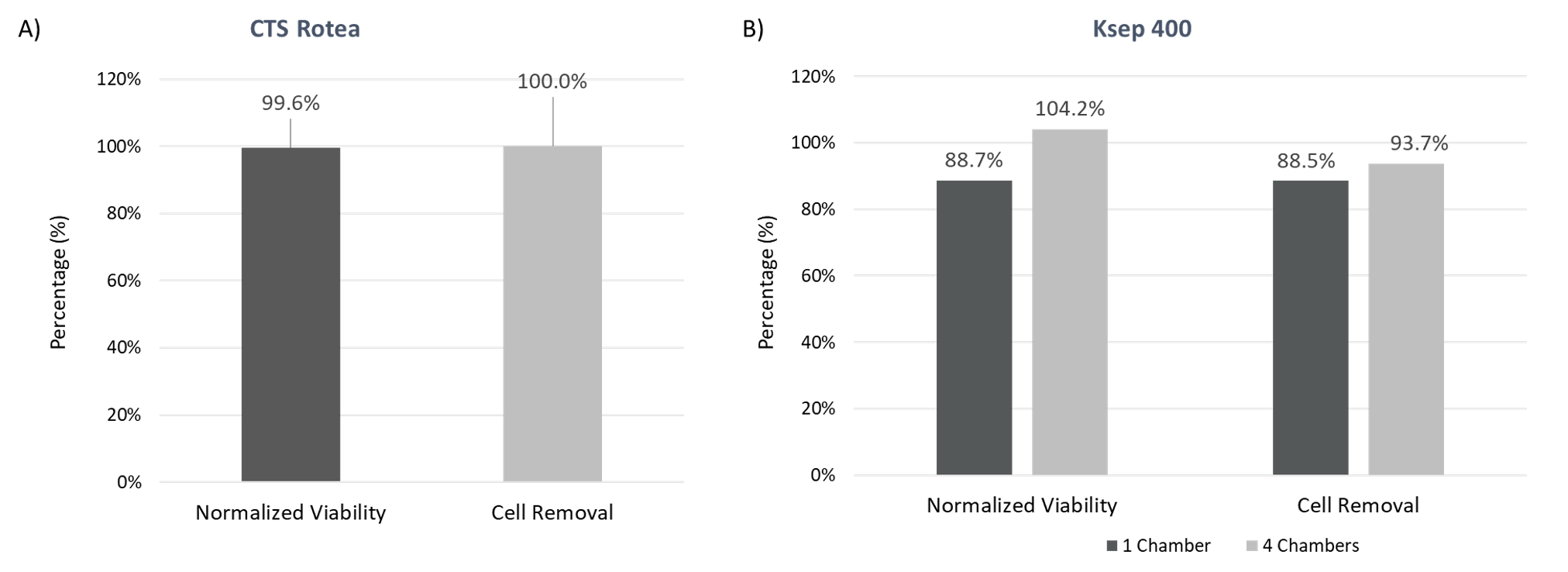
Efficient Cell Removal
Based on the turbidity results, the cell removal from the viral supernatant should be close to 100% and this is indeed the case for both CFC systems (Figure 4) where cell removal from viral supernatant is calculated at ≥88%. The normalized viability shows that the cells removed retain their viability compared to the pre-clarification starting material, despite being very susceptible to cell death after viral production, which limits intracellular protein and nucleic acid introduction into the clarification process. This shows that both CFC systems can effectively remove the viral production cells from the supernatant to prepare for downstream filtration.
-
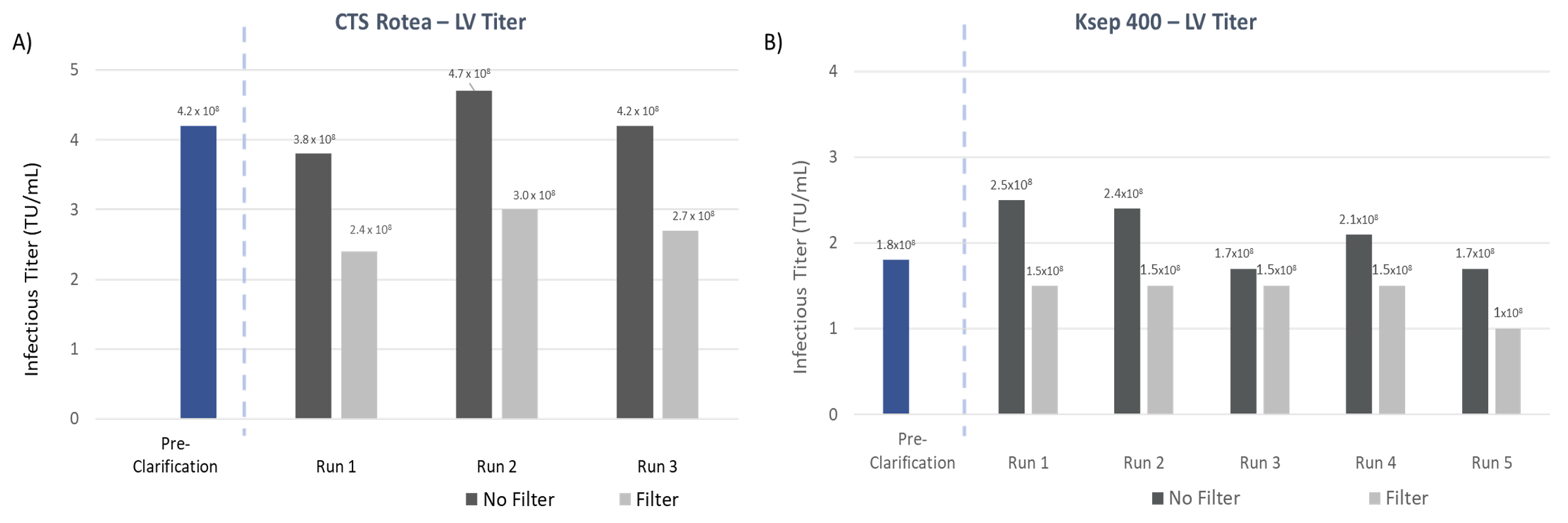
High Lentiviral Vector Titer
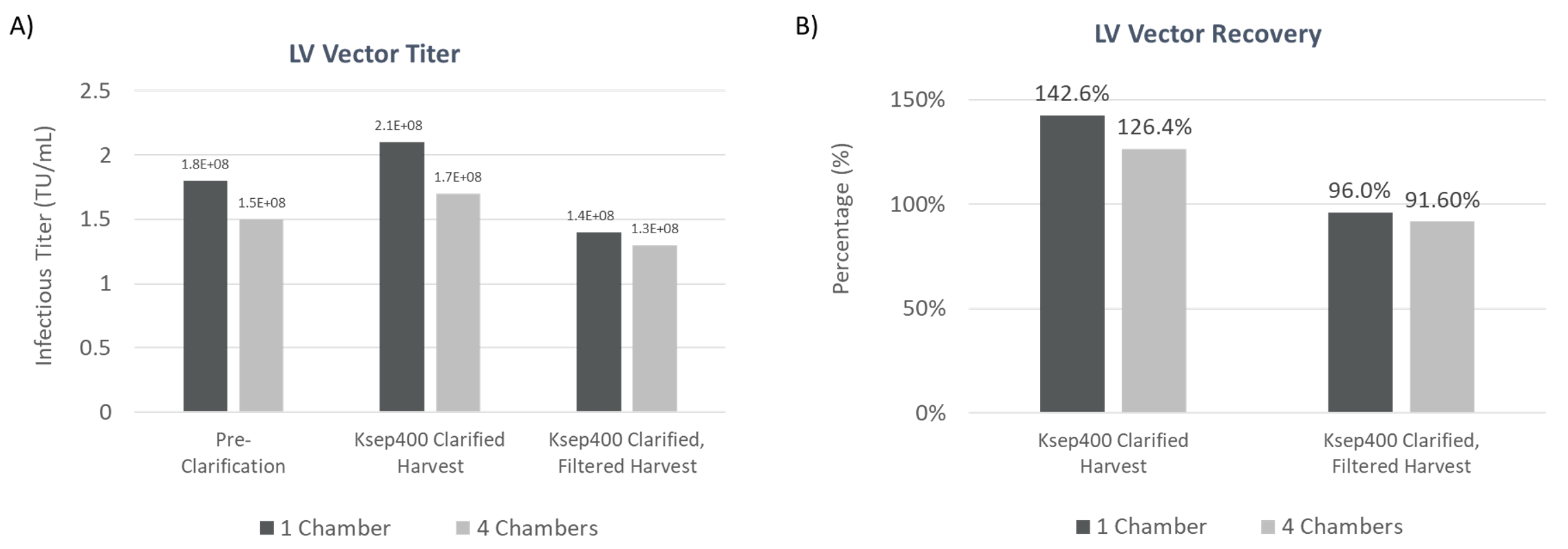
An important objective for clarification is to effectively recover the product of interest—the lentiviral vector, yet low recovery is common in traditional purification schemes where LV yields are commonly less than 50%5,6. The LV titer, quantified by measuring the concentration of infectious particles before and after clarification can be used to determine LV recovery after CFC. In the studies presented here, both instruments were able to maintain high LV infectious titers of >108 TU/mL with LV recoveries of >80% of pre-clarification titer indicating there is no significant loss in infectious titer.
The results are consistent across multiple runs showing high experimental reproducibility. Additionally, comparable LV titer and vector recovery between 1 and 4 chambers for the Ksep 400 shows the consistency and built-in scalability of the instrument (Figure 6). Together, the CTS Rotea System and Ksep 400 provide a effective solution for LV clarification that works for small to large-scale process workflows.
Final Remarks
The demand for LVs will remain high in the foreseeable future as the therapeutic benefits of cell and gene therapy are realized and their clinical utility for new applications are actively being explored. One way to support the industry is through optimization of LV bioprocessing. Here we demonstrate counterflow centrifugation as an innovative and scalable technology, which can unlock a new way to clarify lentiviral vectors. The CTS Rotea System and Ksep 400 jointly offer an automated closed system technology solution to meet clarification demands across a broad range of production scales and overcome the pitfalls of conventional centrifugation strategies. CFC ensures high step yield, facilitates success in post-clarification filtration to maximize the commercial feasibility of cell and gene therapy products and make them more accessible to patients.
For more information, please see
About the Authors
 Carl Dargitz, R&D Manager, Cell & Gene Therapy Platforms, Thermo Fisher Scientific
Carl Dargitz, R&D Manager, Cell & Gene Therapy Platforms, Thermo Fisher Scientific
Carl Dargitz is a Manager in the Cell & Gene Therapy Platforms R&D group developing cell therapy instrumentation at Thermo Fisher Scientific. Previously, he developed reprogramming and characterization assays for PSCs and immune cells at Thermo Fisher. Before joining Thermo Fisher, he worked at the Salk Institute for Biological Studies at the stem cell core facility managing the core’s reprogramming and characterization services. He received his M.S. from California Polytechnic State University, San Luis Obispo in Biomedical Engineering with a specialization in stem cell research in 2013
 Magda Tomala, PhD, Manager of Testing Solutions and Analytics, Separation Technologies Application Testing, Sartorius
Magda Tomala, PhD, Manager of Testing Solutions and Analytics, Separation Technologies Application Testing, Sartorius
Magda Tomala, Manager of Testing Solutions and Analytics at Sartorius, is providing technological and process leadership in the field of viral and non-viral based applications. Before joining Sartorius, Magda worked for Crucell (now Janssen Vaccines) and Novartis, where she held various scientific and managerial roles in the process development areas for Cell Based Vaccines, Biologics and Cell and Gene Therapies. Amongst her involvement in process intensification and technology transfer, she contributed to the successful submissions of several viral vector INDs and the BLA for the CAR-T cell therapy Kymriah, which obtained FDA approval in 2017. Magda holds a PhD degree in Biotechnology / Technical Chemistry from Leibniz University of Hannover (Germany).
