
Optimize Changeover Workflows with ÄKTA readyflux – An Automated, Single-use Crossflow Filtration System
In biomanufacturing process efficiency is key. This is particularly true in pilot and small scale manufacturing where frequent change-overs between product campaigns or batches is often required. Cleaning and validation during change-over can involve significant time, labor, water and utility costs. One way to optimize workflows is to use single-use technologies and another is to automate processes as much as possible.
GE Healthcare has combined these efficiencies in the ÄKTA readyflux, a next generation, automated, single-use crossflow (tangential flow) filtration system for pilot and small-scale manufacturing. The system features a single-use flow path that minimizes the cross contamination risk, reduces the need for cleaning, and shortens the batch change-over time. In addition, automated filtration protocol capabilities in both upstream and downstream applications simplify automation implementation. Automation capabilities include extensive monitoring and control functions.
Filtration Flexibility
The system offers filtration flexibility by allowing the use of both filter cassettes and hollow fiber filter cartridges for microfiltration and ultrafiltration operations. It is also capable of supporting a wide range of disposable bag types.
Compact design for a more flexible facility
The ÄKTA readyflux has a compact design, which improves facility flexibility by utilizing a smaller footprint for filtration. In addition, the system is floor standing and can easily be rolled in and out of the production facility as needed.
The ÄKTA readyflux can also be connected to other single-use equipment. For example, in upstream applications the ÄKTA readyflux can be connected to Xcellerex™ XDR stirred-tank bioreactor systems or rocking WAVE™ Bioreactor systems for use in clarification of cell culture feed. Used together with the ÄKTA ready chromatography system, ÄKTA readyflux can be used for concentration and buffer exchange in downstream applications. Xcellerex XDUO and XDM mixing systems can be used as a recirculation reservoir when working with ÄKTA readyflux.
The innovative design permits low minimum recirculation volume for high concentration factors and product recovery. It is suitable for use in a cGMP environment as well.
Easy Automation of Process
The UNICORN software provides intuitive and flexible method creation, system control and process evaluation to simplify your filtration tasks. By using the phase editor of the UNICORN system control software, automation methods are easily created to your application. An installation wizard with colors assigned to the different flow path sections facilitates Flow Kit installation. To further streamline the process, manual interaction has been reduced by automating the crossflow filtration procedure using UNICORN 7. Method creation has been simplified and an interactive process picture (Figure 1) shows the current flow path, valve positions and monitors values in real-time. The system requires no programming expertise, which permits time to be spent on other activities. Automation reduces operator dependency and adds consistency to the process.
Figure 1:
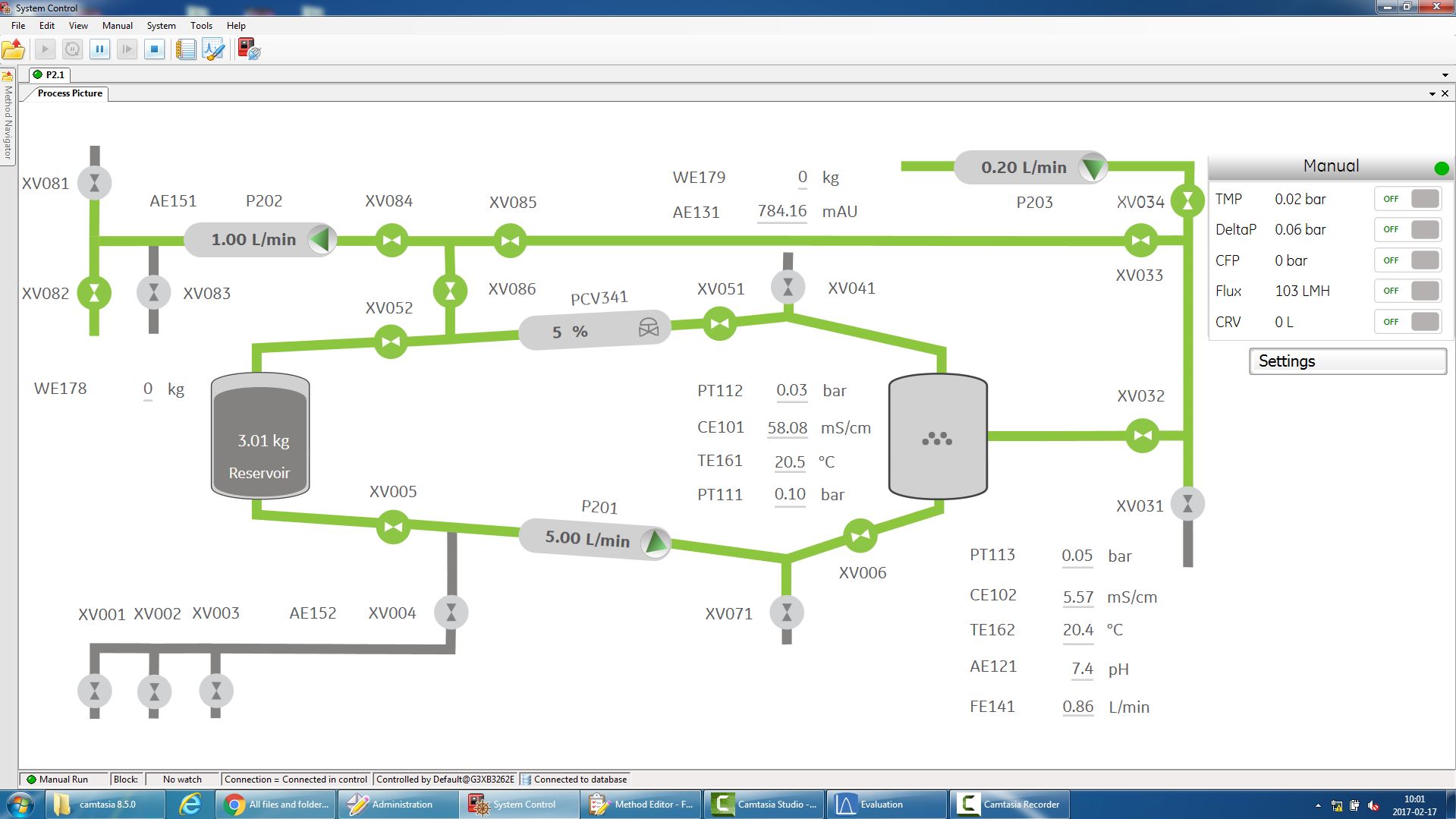
Comprehensive control with UNICORN software
UNICORN system control software is based on an integrated controller and an intuitive user interface. To facilitate handling, the interface uses the familiar Windows® environment. The run sequence is determined by the end-user for control of a specific process. A graphical interface helps create the process sequence or conventional line programming can be performed by advanced users. The UNICORN software contains the tools needed to perform almost any type of cross flow filtration process at different scales, from setting up and running a method to evaluating the data.
The software includes four modules:
- Method Editor: provides an easy interface to create or modify methods.
- System Control: allows performing and monitoring the run in real time.
- Evaluation: supports data analysis and report generation.
- Administration: used to set up user access, view logs, and manage the built-in SQL Server® database tools.
External user audits have shown that the UNICORN development process offers good adherence to the framework, principles, and practices described in GAMP™ 5 and that the functionality of the product is acceptable for use in a cGMP regulated environment in a manner that complies with 21 CFR Part 11. The UNICORN software uses a standard for open platform communication (OPC), allowing for real-time and historical data access as well as third party software control. A Pro bus™ node is available for connection to external programmable logic controller (PLC)/ distributed control system (DCS).
For more information, visit http://bioprocess.gelifesciences.com/aktareadyflux
