Optimization of a Protein A Chromatography Process for a Herceptin® Biosimilar (Trastuzumab)
Utilizing a Protein A Resin Platform Approach for Purification of Antibody Fragments and Single Domain Antibodies Reduces Process Time
A Guest Blog by:
Platteau, Gerald¹*; Ströhlein, Guido¹; Gaspariunaite, Vaiva²; Vincke, Cécile²; Sterckx, Yann²; Muyldermans, Serge²
Introduction
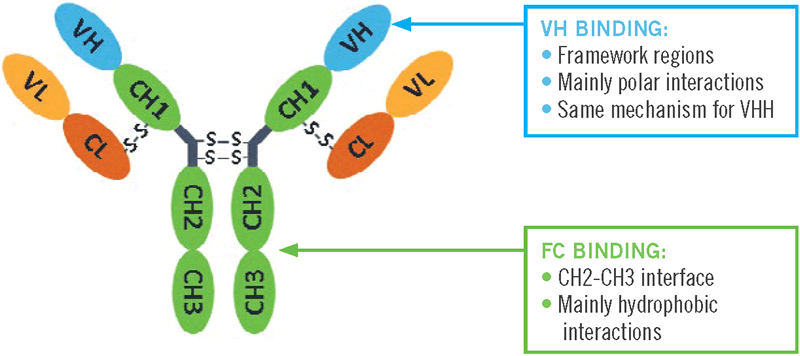
The standard capture purification of full-size, classical antibodies is typically performed with Protein A affinity chromatography. Binding of the Protein A affinity ligand to the “Fc” region of antibodies (Abs) (abbreviations are defined at end of paper) takes place at the juncture of the constant domains 2 and 3 of the Ab heavy chains (Lewis et al. 2008). This high affinity binding primarily involves hydrophobic interactions. Antibodies that belong to the same subclass have greater than 95% homologous Fc-regions, allowing Protein A to be a capture platform for a wide range of antibodies (Ghose et al. 2005).
Following the wave of successful commercial monoclonal antibody products, various forms of antibody fragments are now becoming an important class of next-generation therapeutic proteins. This includes Fabs and fusion proteins of the Fab variable domains. From the variable domain of the heavy-chain antibodies of camelids, the VHH sdAbs have been derived. These VHHs represent some of the smallest antigen binding antibody-derived proteins. As such they are more stable than full size mAbs, can be produced in microbial organisms, and offer higher target binding events per gram of product. Due to the lack of an Fc region, these antibody fragments cannot be captured with most engineered Protein A affinity ligands. However, Amsphere™ A3 Protein A ligand exhibits a high affinity for VHH single domain antibodies (Figure 1).
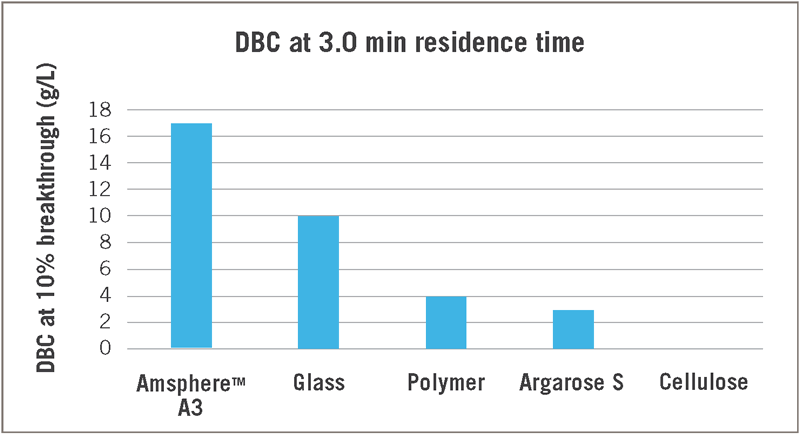
Compared to 4 other commercially available PrA resins, Amsphere A3 has the highest DBC. The resin that comes closest has a 40% lower DBC, and given its glass matrix, is not as caustic stable. Results in Figure 1 were obtained with a default protocol for Protein A affinity purification, which included VHH elution at pH 3 (Table 1).
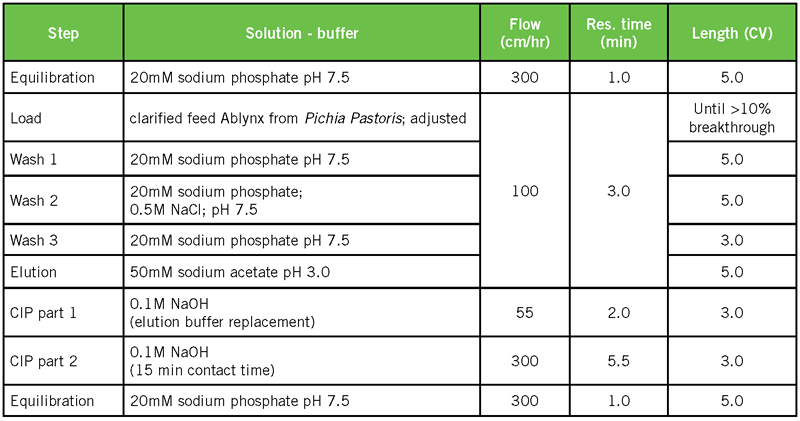
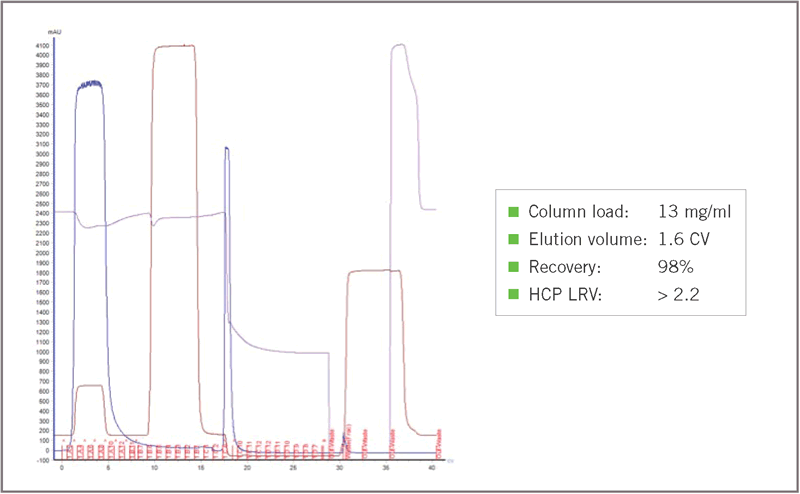
Figure 2 shows the chromatographic performance of a batch purification run of the VHH with Amsphere A3, demonstrating a low elution volume with good protein recovery and HCP clearance.
It is of interest to delve further into the interaction between the Amsphere A3 ligand and the VHH sdAbs which make the results in Figure 1 possible. Since binding to the Fab region of the mAb Herceptin® was also demonstrated (data not shown), the mechanism of binding to the VH domain was also studied. Conditions for binding of VHH to Amsphere A3 were evaluated. DBC data for VHHs were compared to those for mAbs. The use of Amsphere A3 for antibody fragment capture was compared against other currently available resin types. Finally, the range of antibody fragment variants for which Amsphere A3 can be used as capture resin is described.
Binding sites in the Amsphere A3 ligand and VH(H) molecules
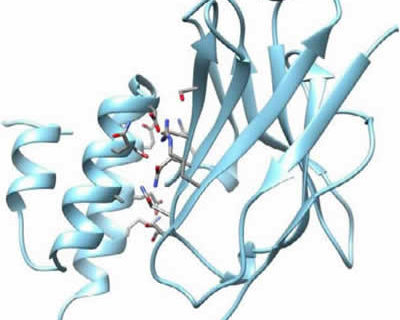
In collaboration with the Laboratory of Cellular and Molecular Immunology at the Vrije Universiteit Brussel (VUB), the crystal structure of a VHH-PrA complex was obtained. Figure 3 provides a schematic overview of the different steps in the process to obtain the crystal structure.
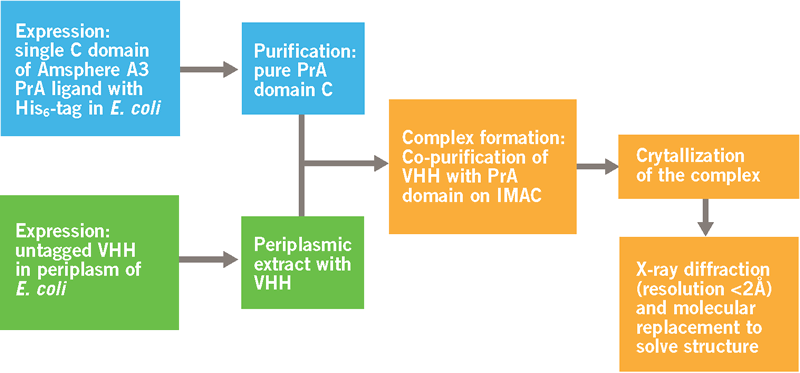
The process (Figure 3) resulted in the crystal structure shown in Figure 4. The structure of both proteins was resolved to < 2 Ångstroms and is well defined in the complex. On the left, we see the 3 helices of the Protein A monomer. On the right, we have the typical structure of a VHH, with 2 sheets of 4 and 5 ß‐strands respectively. The sheet with 5 ß‐strands corresponds to the side in a VH that normally interacts with the VL domain in a classical antibody. The 4 ß‐stranded sheet is solvent exposed and does not participate in antigen recognition. The 3 CDRs are the loops on top of the VHH molecule.
Binding sites for the VHH are located in helix 2 and 3 of the Protein A ligand. 7 amino acids in the Protein A are identified as most important for the interaction with the VHH. Where the binding sites for the Fc region are in helix 1 and 2 and a glutamine in helix 2 is the only amino acid involved in both Fc and VHH binding (Graille et al. 2000).
Interaction sites for Protein A are located in framework regions 1 and 3 of the VHH. This is the side of the sheet with 4 β‐strands. 8 amino acids of the VHH face the Protein A domain and these are mainly polar and/or charged residues: Ser17, Arg19, Lys65, Thr69, Ser71, Gln82, Asn84 and Ser85
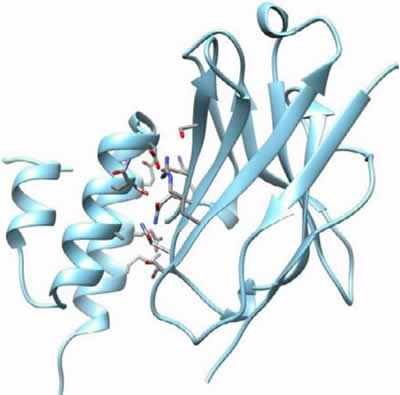
In order to verify whether there is a similar mechanism for Fab binding, an overlay was made of the VHH used in this project, with the published crystal structure of the VH domain of a human Fab fragment (Figure 5). It is clear that these 2 domains have an excellent overlap. All amino acids interacting with the Protein A in the VHH are shared between the VHH and this VH domain. All these amino acids were also conserved in the VH domain of Herceptin (sequence alignment not shown). This strongly suggests a similar binding mechanism for both VHH and VH domains. In the VH domain of a classical antibody, the Protein A-binding region does not interact with the VL domain and is not involved in antigen binding.
Operating conditions
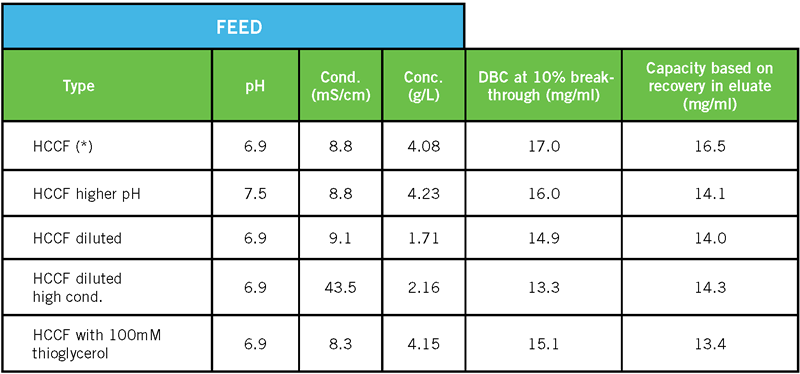
For a VHH sdAb, a range of binding conditions was tested. Related chromatography conditions are the same as in Table 1. Only the conditions of the feed were modified, as shown in Table 2. It should be noted that these conditions, representing a range of feed pH (6.9 – 7.5), conductivity (8.3 – 43.5 mS/cm) and target concentration (1.7 – 4.2 g/L), all resulted in significant DBC values.
Since the interaction with Protein A is based on charged residues, the interaction was expected to be salt dependent. It was therefore double checked if the VHH would elute at high salt concentration (Table 3 and Figure 6).
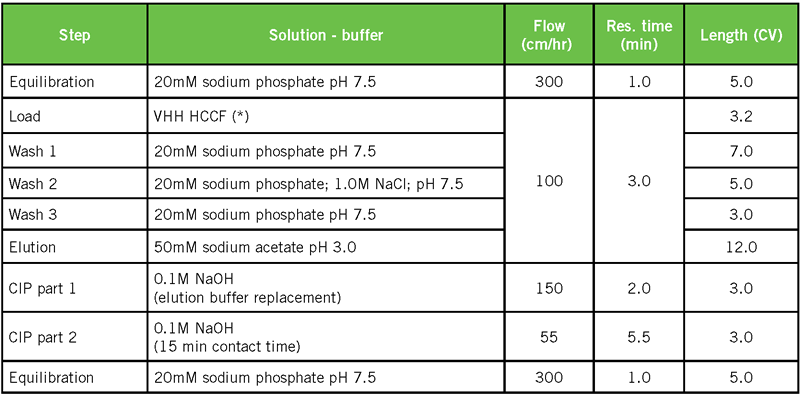
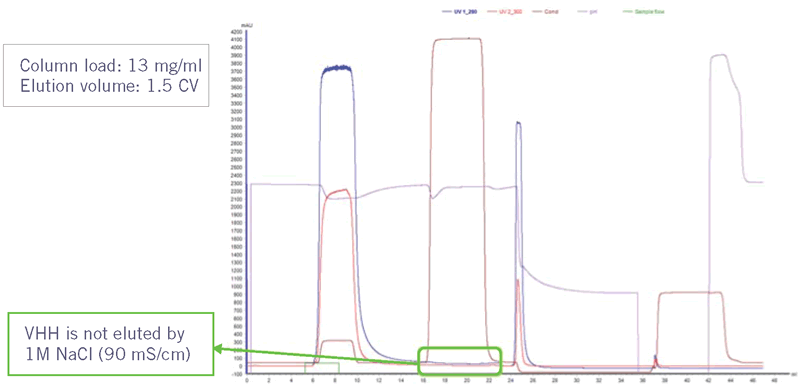
The sdAb is still bound to the column after applying a wash step with 1M NaCl. The binding of a Fab fragment, created by papain digest of Herceptin, was found to be salt dependent (data not shown). It is therefore recommended to use low conductivity conditions (< 5.0 mS/cm) for initial binding studies of VH domain-related targets, but possibly scout higher conductivity conditions.
Evaluating and interpreting DBC values
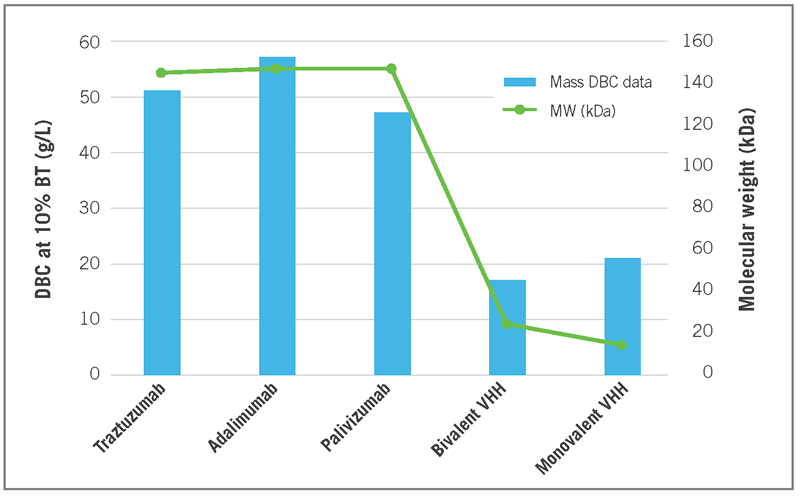
Compared to full size mAbs, the g/L capacity values shown for monovalent and bivalent VHHs are about 2-3 times lower (Figure 7). However, the MW of a full size IgG (approx. 150 kDa) is about 10 times larger than that of a monovalent VHH (approx. 15 kDa). The data in Figure 7, reflects a 5 minute residence time for the mAbs and only 1 minute for the VHH targets. No significant difference was found in DBC at 1 versus 5 minutes for the smaller VHH targets.
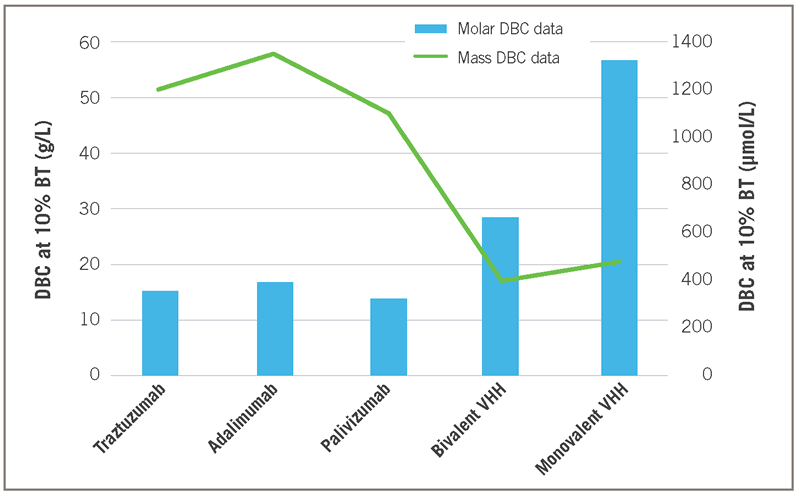
The Figure 7 equivalent molar DBC data, representing the number of molecules that are bound to the resin, expressed in μmol/liter are given in Figure 8. About 3 to 4 times more monovalent VHHs bind per multimeric Protein A ligand than for mAbs. Such results may reflect steric hindrance spatial limitations regarding binding of multiple targets per Protein A ligand.
Comparison to other resins for antibody fragment capture
Two large resin categories are currently used for antibody fragment capture. Ion exchange and mixed mode resins separate the target molecules from impurities based on their surface charge at a given pH and salt concentration. With these resins it is possible to obtain higher binding capacities. However, the advantage of Protein A compared to these resin types is that the process development time is much shorter. Protein A chromatography requires shorter process development work. Also, since it is an affinity step, purity is already very high after the capture step, combined with product recoveries over 95%. Linked to the limited process development is the fact that affinity chromatography has the potential for use as a platform capture step.
The second resin category are affinity resins produced specifically to bind a certain antibody domain, like Protein G or Protein L (Rodrigo et al., 2015). Amsphere A3 has the advantage over these resins of good caustic stability, allowing a high number of runs and thus lowering the overall cost per gram purified product. It may also allow target elution at higher pH (e. g. 3 versus 2). Table 4 gives an overview of chemical stability results related to exposure times of Amsphere A3 in different sodium hydroxide concentrations. Depending on the desired CIP and sanitization regime, the resin lifetime in number of cycles can be computed from the data in the table. The 80% DBC retention is given as it is often used as an endpoint for determining useful resin lifetime.
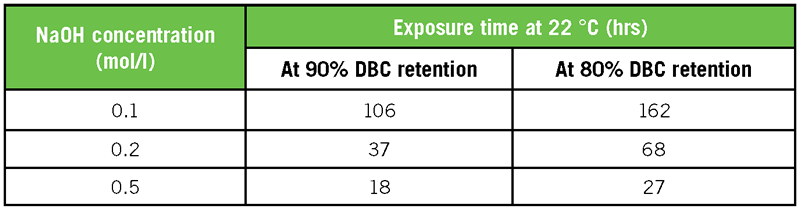
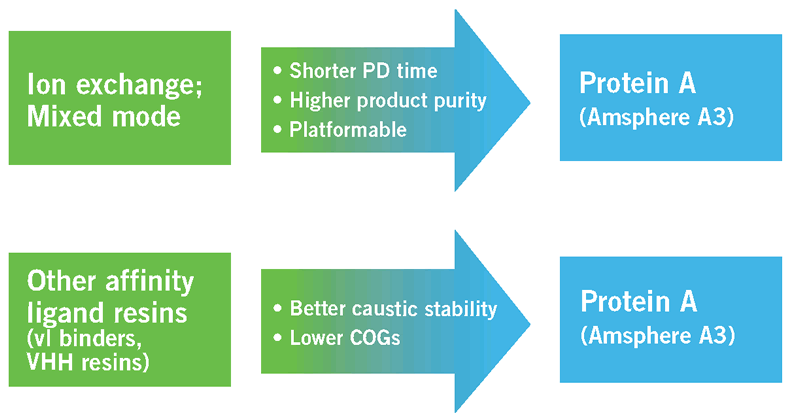
Figure 9 summarizes the above noted advantages of Amsphere A3 over other resin types in regard to primary capture of antibody fragments.
Platform scope for antibody fragment capture
The studies noted above provided some insight into the molecular basis of binding of Amsphere A3 to VH(H) antibody formats. Figure 10 summarizes the 2 variable domain binding targets for Amsphere A3.
The literature reflects binding of Protein A to VH is restricted to molecules of the human VH3 subfamily, with no examples from any of the other gene families. In almost every case, reduced or eliminated binding can be linked to mutations in the described residues. Specifically for VHH sdAbs, 110 molecules were screened in collaboration with Ablynx NV, of which 99% showed binding to the Amsphere A3 Protein A ligand. The fact that the interacting residues are in the framework regions, (so not involved in antigen binding), and that there is no interaction with VL in a Fab, opens the possibility of mutagenizing non-Protein A interacting Ab fragments into binders (Fridy et al. 2015; Henry et al. 2016).
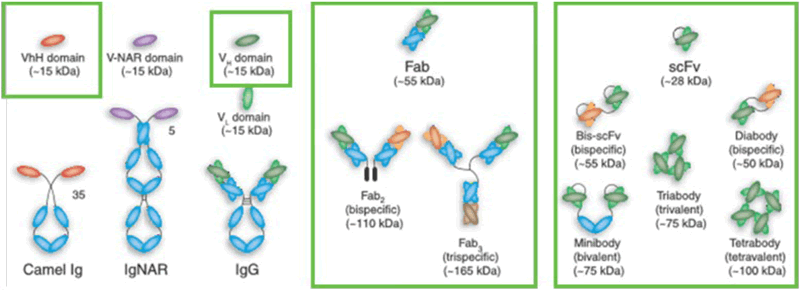
Figure 11 shows the various forms of antibody fragment formats for which Amsphere A3 can be a suitable capture resin. These can be divided into 3 major categories: VHH single domain antibodies, and constructs containing a VH3 domain like Fabs and scFvs.
Conclusions
Besides the use for Fc containing antibodies and constructs, the Protein A resin Amsphere A3 can be used for purification of antibody fragment formats, like the VHH single domain antibodies, and constructs containing a VH3 domain like Fabs and different fusion proteins such as single chain variable fragments. An overview of these binding targets is given in Figure 12. The well-known robustness, high selectivity, and scalability and regulatory acceptance of Protein A based resins make Amsphere A3 an ideal platform technique, minimizing DSP development time.
Related Articles:
Abbreviation list
Ab: Antibody
CDRs: Complementarity-Determining Regions
COGs: Cost Of Goods
DBC: Dynamic Binding Capacity
DSP: Downstream Processing
Fab: Fragment Antigen-Binding
Fc: Fragment crystallizable
HCCF: Harvested Cell Culture Fluid
HCP: Host Cell Protein
IgG: Immunoglobulin G
kDa: kilodalton
LRV: Log Reduction Value
mAb: Monoclonal Antibody
MW: Molecular Weight
PD: Process Development
PrA: Protein A
scFv: single-chain variable fragment
sdAb: Single Domain Antibody
VH: Variable domain of the Heavy chain
VH3: Variable domain of the Heavy chain of the gene family 3
VHH: Variable domain of the Heavy chain of a (camelid) Heavy chain antibody
VL: Variable domain of the Light chain
