
Scale up with confidence – Introducing ReadyToProcess 32 L (450/200)
To reduce costs, increase flexibility and shorten time to market, the use of single use and disposable technologies have increased significantly in biopharmaceutical development and manufacturing.
In downstream processing prepacked chromatography columns reduce the need for time consuming cleaning validation and column packing. The last few years has seen a steadily increasing implementation of prepacked chromatography columns in process development and clinical manufacturing. Many of these projects are now scaling up for commercial production.
To meet these needs GE Healthcare is now introducing ReadyToprocess™ 32 L (450/200) for manufacturing-scale purification of biomolecules from bioreactor harvests of up to 2000 L
ReadyToProcess columns are delivered prepacked, prequalified, and presanitized to enable significant time savings in biopharmaceutical production.
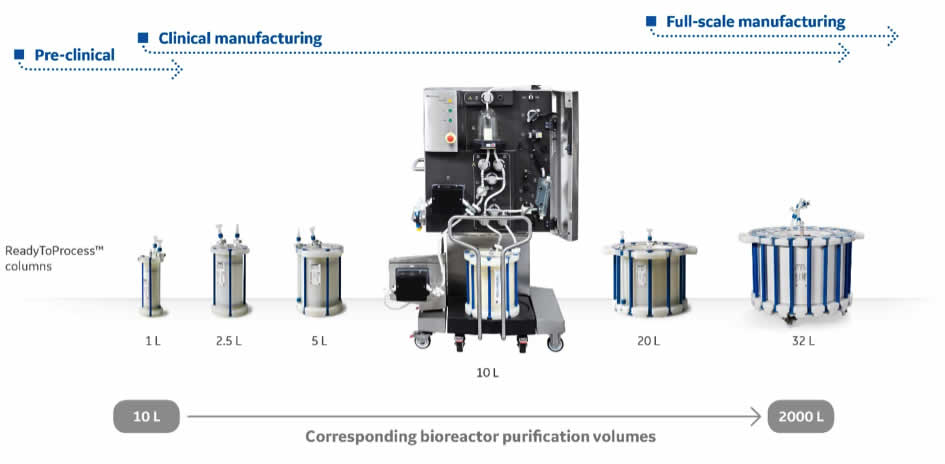
The column design as well as robust and validated packing methods enable production with high lot-to-lot consistency. The standardized column format allows for off-the-shelf availability for short delivery lead times. ReadyToProcess columns are covered by our extensive security of supply program for chromatography resins, with customer safety stock possibilities.
Additional bed heights and resins on the ReadyToProcess column portfolio are available through our services for custom resins and columns.
For more information, visit https://gelifesciences.com/ReadyToprocess32L
