UPDATED – A New Model for Continuous Processing in Downstream Purification
Continuous processing has certainly been a hot topic of late, and while most companies are not ready to move to a fully continuous process, many are investigating how incorporating components of continuous processing could improve their manufacturing.
Why Continuous Processing?
In the biopharmaceutical industry there is an ever-present drive to increase product yield and reduce cost. Since the inception of biopharmaceutical manufacturing in the 1980’s there have been continual improvements to the process. These improvements have culminated in the most common approach to biopharmaceutical manufacturing, fed batch suspension culture, most commonly expressed in CHO cells. Improvements have continued in areas including advancements in cloning, media formulation, removal of animal components, and downstream purification resins and columns, but the fed batch production flow has remained.
While a big move away from fed batch at this time is unlikely for many reasons, the industry has been more actively considering the continuous processing paradigm and a few companies have incorporated these methods or components of it. Continuous processing offers attractive potential benefits including reduction of costs, increase in product throughput, a smaller footprint, and more facility flexibility, among others.
As the interest in continuous processing has increased, suppliers have begun to offer technologies to enable continuous manufacturing. One of these is in upstream perfusion culture, which we have covered several times in past blogs. Another area is in downstream purification. These continuous processing tools can be used to improve manufacturing as stand-alone solutions or can be incorporated with other technologies to create a fully continuous process.
Continuous mAb processing using Integrated Single-Use Purification Technologies
At the BioProcess International Conference (BPI) in October, Michael Phillips, Director, Bioprocess Development, EMD Millipore gave a presentation titled, “Integrated Single-use Purification Technologies Enabling Continuous mAb Processing.” The talk covered EMD Millipore’s development of a process to enable continuous mAb purification. The components of the process can be used in conjunction with other continuous processing operations or as stand-alone solutions.
The goal was to develop a process for mAb purification that addressed key needs of biomanufacturing customers, which included increasing manufacturing speed, reducing drug costs, improving facility flexibility and reducing operating and capital investment risk.
EMD Millipore’s development team began their work by evaluating and/or developing over 10 capture technologies and over 40 purification chemistries. After the assessment they developed a process that integrated the best of their evaluations, offering technologies that made changes to the traditional clarification, capture and purification steps. Technologies they incorporated included:
- Precipitation-based clarification and solids removal using novel depth filtration media
- Continuous multi-column Protein A chromatography capture
- Fully connected flow through purification train that was developed for purifying Protein A elution pools
Figure 1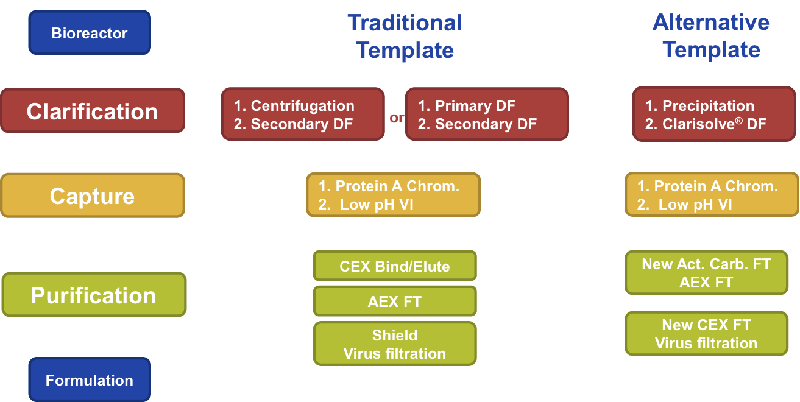
As a result of much development work, EMD Millipore created an alternative process template using integrated technologies that could either be used to support traditional batch processes and that could also enable downstream continuous processing of mAbs. (Figure 1)
Key features of the Alternative Process
Clarification
During the clarification step, EMD Millipore employed a combination of precipitation-based clarification and their Clarisolve® depth filter. This allowed for a fast and easy way to clarify without a centrifugation step.
Capture
After evaluation it was determined that Protein A chromatography was best for mAbs, but productivity could be increased and flexibility augmented by employing multi-column continuous chromatography using incompressible Protein A resins. The result, in a follow up case study, was a 10-fold increase in productivity and no deleterious effects on product purity.
Purification
In looking at purification options, much time was spent on flow through chemistry evaluation and selection. EMD Millipore looked to identify chemistries with selective host cell protein binding, while understanding the effect this had on the overall process. Considerations included mAb yield, viral clearance, and purification. An effort was also made to minimize solution condition modifications and necessary adjustments.
In developing the process, two new adsorber technologies had to be created to meet the needs of the flow through purification. The first step in the flow through purification process was a new Activated Carbon-based adsorber that utilized charge and sized based selectivity. It was designed to target smaller host cell proteins, leached Protein A and cell culture medium additives such as anti-foam, surfactants, antibiotics, and insulin. The new adsorber offered high binding capacity and effective removal of cell culture ingredients while maintaining high product yield.
The next step was to use an AEX adsorber to capture larger acidic host cell proteins, DNA and viruses.
Lastly another new adsorber, CEX FT adsorber, was specifically developed to enable the flow through removal of mAb aggregates at high product loadings. This adsorber offered the ability to selectively bind mAb aggregates and allowed for high mAb loading. Another advantage was that since it is mAb independent and no bind-elute steps were needed, both the process development time and buffer requirements were greatly reduced.
The three steps work synergistically to provide key flow-through purification advantages including:
- Reduced number of intermediate solution adjustments required and only one pH change
- Binding the impurities permitted smaller media volumes, facilitating the use of single-use technologies
- No bind-elute steps, thereby significantly reducing process development time and enabling continuous processing
Results of the New Process
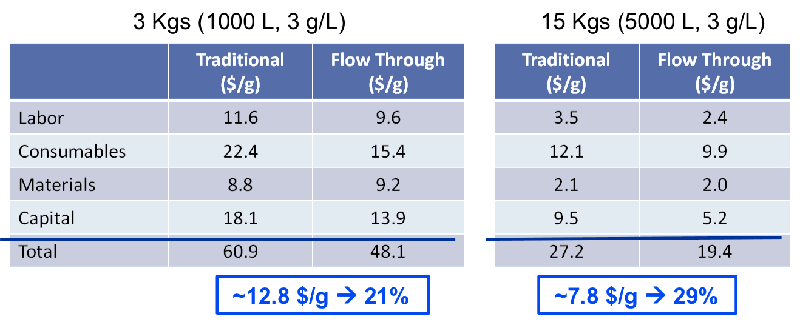
After completing the new process template, success and key benefits needed to be assessed. A case study was conducted that compared yield, host protein, DNA, leached Protein A removal and product quality between the traditional process and the alternative process. Based on the study results, the new process was found to be comparable in all above areas, however the new process resulted in fewer product aggregates in the final pool and was a more economical option. Overall, process modeling indicated a significant reduction in cost of goods (see figure 2).
Figure 2
Key Benefits
Increase in Speed of Manufacture
Speed was increased via higher throughput operations, which required lower cycle times. In addition single-use technologies reduced cleaning and validation efforts, thus lowering process downtime. Lastly, the elimination of the bind-elute steps reduced process development efforts.
Cost Savings
Primary cost savings drivers were gained by the following:
Reduced Capital Investment
- A reduced number of intermediate solution adjustments and only one pH change allowed for the elimination of many intermediate pool tanks and subsequent intermediate tankage equipment. This also allowed for fewer process skids.
- Incorporating the use of more single-use technologies required less capital investment in equipment
- Reduction in the number and size of buffer tanks
Decrease in the Cost of Consumables
- Reduced buffer and water consumption
Reduction in Labor Costs
- Increase of single-use technologies result in less time spent cleaning thus lowering labor costs and lower water usage and resulting in less manufacturing downtime
- The elimination of the bind-elute steps resulted in less process development work
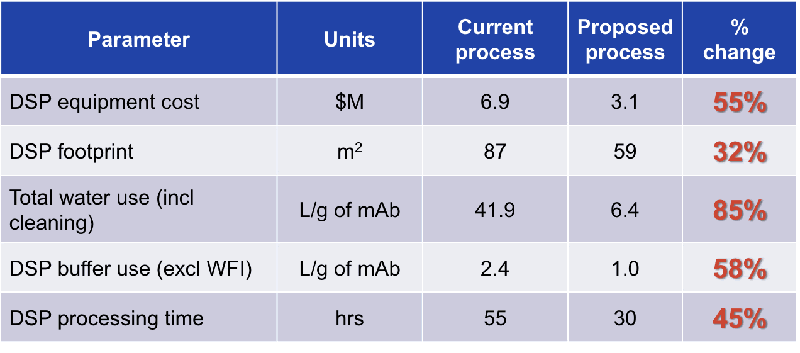
A good representation of the savings by type can be seen in Figures 2 and 3.
Figure 3
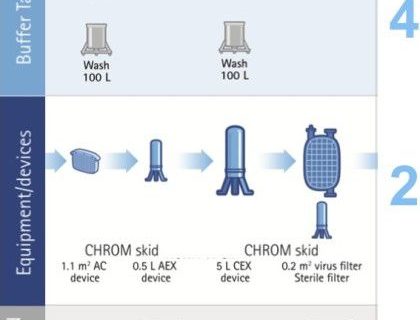
Facility implications
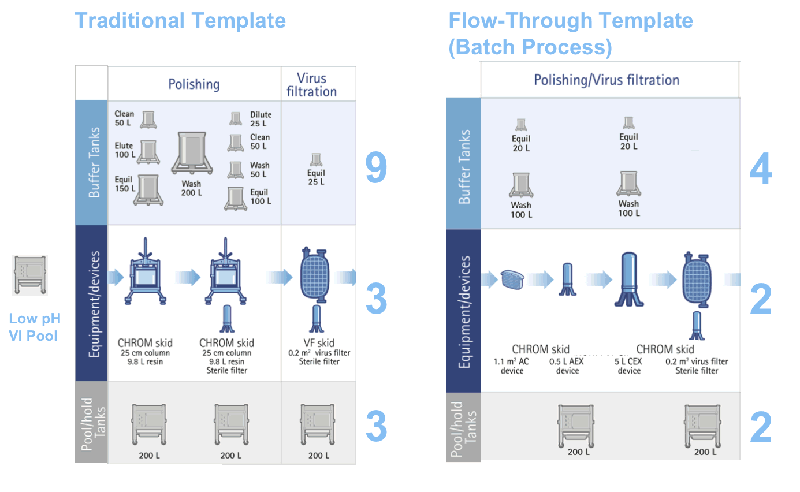
As seen in Figures 3 and 4, the benefits of the flow through technologies to facility utilization include:
- Smaller overall footprint through the elimination of and use of smaller buffer tanks (4 buffer tanks versus 9 for the traditional template).
- More facility flexibility by reducing product hold tankage and employing single-use technologies.
- Improved facility utilization and multi-product facility use.
Figure 4: Improved facility implications for the new flow-through template
Reduced Risk
Risk is reduced in two ways, both in operating risk by utilizing closed bioprocessing and in capital risk by lowering up front capital investments.
Conclusion
This article has provided one example of the work being done to enable continuous processing and there are several others. Although many biomanufacturers are not looking to move to a fully continuous process at this time, there is much to be gained from incorporating specific enabling technologies to increase productivity of a process and/or facility or in specific applications. Continuous process enabling technologies like single-use technologies, perfusion culture in upstream and flow-through purification in downstream may provide long term solutions for improving biomanufacturing through continuous processing or may provide a clever solution for a portion of the traditional biomanufacturing process. In either case, these innovative technologies are definitely worth further investigation.
To learn more, a detailed presentation of these technologies is summarized in the following book chapter:
Gillespie, C., Kozlov, M., Phillips, M., Potty, A.S.R., Skudas, R., Stone, M., Xenopoulos, A., Dupont, A., Jaber, J., Cataldo, W., (2015) Integrating Continuous and Single-Use Methods to Establish a New Downstream Processing Platform for Monoclonal Antibodies. In: Subramanian, G. (Ed.), Continuous processing in biopharmaceutical manufacturing. Wiley-VCH.
This article was updated April 17, 2015 to add additional details and resources.
