Affordable Biologic Downstream Purification with Single-Use Protein A Membrane
At this year’s Boston Biotech Week there were many exciting talks on downstream purification and associated new technologies. In particular, there were several talks about optimizing the downstream purification process. One very interesting talk, given by Renaud Jacquemart, PhD Principal Scientist, Director Vaccines Process Sciences, was titled “Enabling Manufacturing Of Affordable Biologics Through The Use Of A Protein A Membrane In A Single-Use Purification Strategy ” and focused on the application of a fully single-use chromatography purification process in place of resins. This strategy envisions the use of a unique Protein A membrane for which Natrix recently signed collaboration agreements with Merck & Co. and Sanofi.
Creating a more affordable purification strategy
In his talk, Dr. Jacquemart begins by talking about the goal of creating a more affordable purification strategy and how the Natrix approach incorporates a holistic vision of the entire manufacturing process. To meaningfully decrease total cost and create the most efficient process, companies must significantly reduce the physical scale of manufacturing facilities and enable greater flexibility. This permits faster turnaround and accommodates a wider range of scales and products for any given time period. Achieving these goals requires a large increase in productivity and much-simplified single-use architecture for purification.
Dr. Jacquemart then presented a collaborative project in development with Univercells. The goal of the collaboration is to create a continuous, automated, small footprint manufacturing facility. To achieve this, the focus will be to combine upstream and downstream processing in one small footprint. By incorporating improved productivity in each unit operation, smaller facilities are enabled. The facility capital investment is estimated to be less than $25 million all-in for a mAb production capacity of up to 500 kilograms per year with cost of goods on the order of $50 per gram. A huge benefit of this continuous, small footprint manufacturing concept is that it would enable manufacturing in or near smaller emerging markets at costs that enable a sustainable economic model.
Next Dr. Jacquemart outlined the specifics of the manufacturing process concept. Univercells provides perfusion-based, small footprint, high density upstream manufacturing. Natrix’s role is to enable the development of a fully single-use platform-able downstream process with up to a 30-fold increase in productivity
Natrix’s priorities include:
- High productivity throughout the process to shrink the manufacturing footprint.
- Forward thinking to meet current and future trends.
- Simple to implement and operate, as the process is automated.
- Process architecture compatible with most mAb processes, but also with other proteins and viral vaccines.
In developing the high productivity downstream process, Natrix considered several key issues. As described by Dr. Jacquemart, this included the thought that although Protein A resin is the gold standard for mAb capture, it has limitations considering the current state of upstream productivity, i.e. higher titers and proven intensification techniques. The challenge in using columns include relatively low productivity driving a large footprint, reduced flexibility, and re-use requirements for amortization that drive higher labor and validation costs. These limitations drive high capital and operating expenses, and resins must be cycled many times over many batches to realize cost-effective amortization. They also identified that the low productivity of the Protein A resin capture makes it difficult to achieve an efficient continuous process, as the purification systems become complex and matching upstream/clarification throughput is also difficult.
To address these issues, Natrix is developing a Protein A capture membrane, to provide the following benefits:
- Up to 30X the productivity of resins (6 second residence time, up to 50mg/mL DBC at 10% breakthrough)
- Good manufacturing reproducibility
- Flow rate and cycling flexibility to enable matching the upstream and clarification throughput
- Small columns enable single-use per batch with up to 200 cycles to amortize the full life of the Protein A media
- Plug and play solution with pre-packed membrane columns suitable for use with most chromatography system platforms
- Typical caustic stability performance that enables sanitation flexibility
- Demonstrated purification performance with 20 different antibodies at bench scale
They are also considering incorporating a multi-column strategy like GE Healthcare’s AKTA PCC, Pall Cadence BioSMB, or Benoit Mothes’ ASAP concept (Sanofi Vitry) in order to make this process fully continuous, but this has not been tested yet since the membranes deliver sufficient productivity using traditional chromatography methods.
Dr. Jacquemart then showed a specific example of a common biosimilar monoclonal antibody used to test this manufacturing method and summarized the key findings from studies performed with more than 20 mAbs:
Antibody:
IgG1, 142 kDA, pl 8.2, produced in CHO cell culture to approximately 5 g/L (Ab) and approximately 2×106 ng/ml (host cell protein)
Challenges: Prone to aggregation at low pH (HMW >10%)
Protein A run conditions:
EQ: 20mM sodium phosphate, 150 mM NaCL, pH 7.6
Elution: 50 mM Sodium Acetate, pH 3.5
Strip: 0.1 M acetic acid, CIP 0.1 M NaOH
The Protein A membrane was able to clear HCP to <1500ppm (2.5 LRV) from harvest, or to <100ppm (3.5 LRV) after high performance clarification.
A holistic approach
While most companies still consider optimizing upstream and downstream separately, the Natrix – Univercells team employs a holistic approach where all aspects are considered together in order to obtain the best compromise. State of the art clarification technologies were therefore evaluated during this holistic optimization, not only for their particle and cell debris removal capability but also for the benefits they provide to the subsequent chromatography steps. Consequently, various techniques like precipitation, flocculation, TFF and charged depth filtration were assessed. Some of the strategies provided significant HCP reduction (2-3x), and the protein A membrane was able to clear HCP to <1500ppm (2.5 LRV) from simple dead end filtration, or to <100ppm (3.5 LRV) after high performance clarification (optimum performance achieved with Millipore D0HC and X0HC charged depth filters).
Key highlights of the membrane technology are captured below:
Process Economics
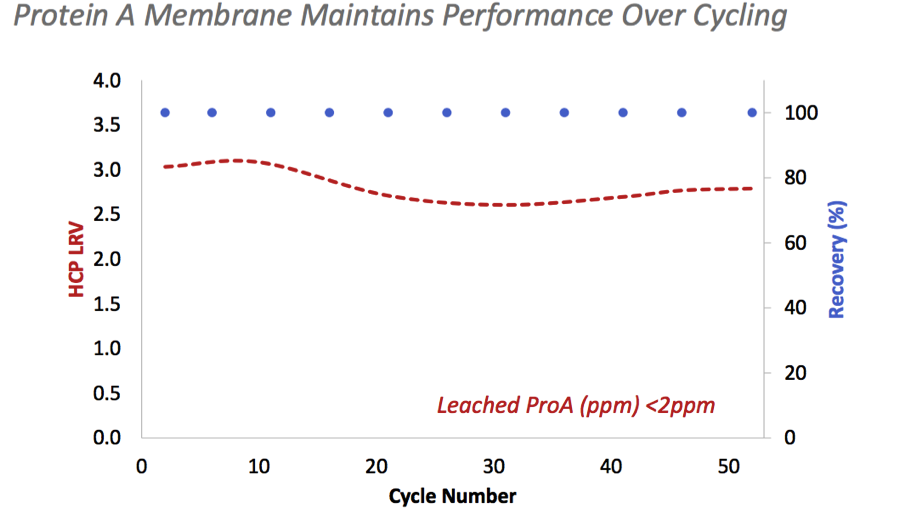
Protein A Media life could be fully utilized in a single batch to optimize the process economics because the membrane maintains its performance over cycling (Figure 1).
Figure 1:
Comparable to the Reference Process
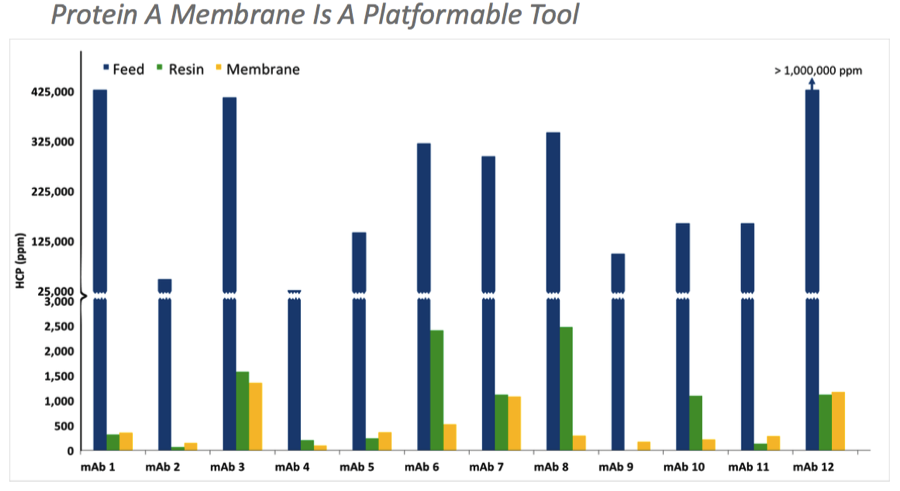
Protein A membrane clearance is comparable to reference Protein A resins and was effective across a number of mAbs, thus demonstrating its use as a platform process (Figure 2).
Figure 2:
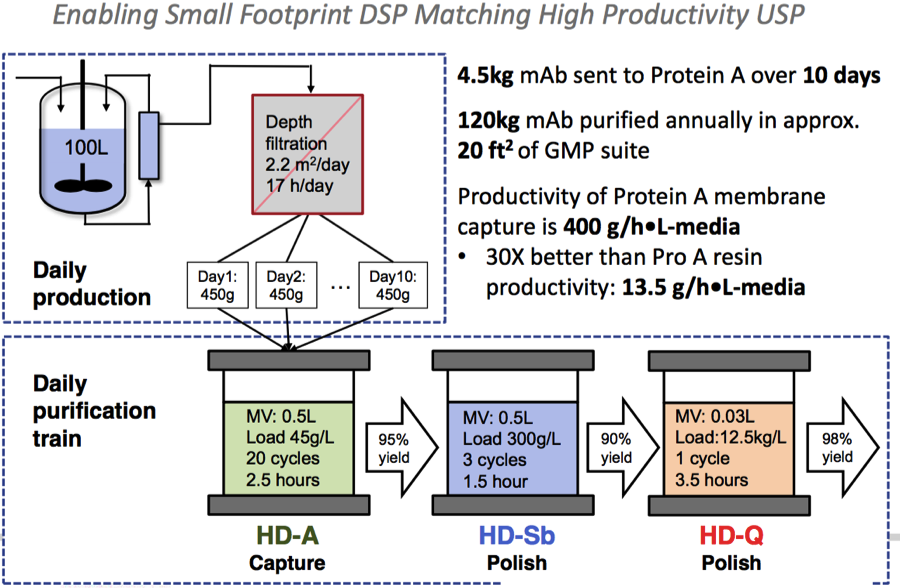
High Productivity
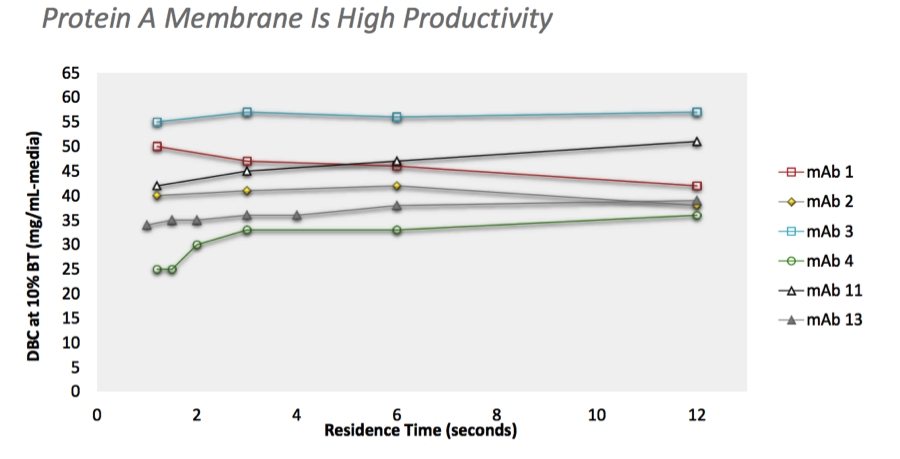
Using a high productivity protein A membrane enabled right-sized columns to match the upstream process throughput in a small footprint, flexible manufacturing facility (Figure 3).
Figure 3:
Small Footprint Downstream Processing
Dr. Jacquemart then showed a slide adapted from a scientific article1 (Figure 4) published by the team earlier this year in CSBJ, which provided a visual representation of the downstream process.
Figure 4:
Dr Jacquemart completed his data review by showing intermediate and final polishing step results for the example antibody. Natrix’s mixed mode cation exchanger membrane reduced the aggregate level to 0.5% while the anion exchanger reduced the HCP level to below the threshold acceptable for use in humans, with no detectable DNA and 7LRV MVM viral clearance up-to a load of 20 kg/L.
Summary
In summary, Dr. Jacquemart’s stated that Univercells and Natrix have been able to design a manufacturing process that provides a very large reduction in footprint and capital investment and that substantially reduces antibody cost-of-goods. The Natrix HD membrane enabled this high throughput, sequential continuous downstream process by matching the productivity of the upstream process. In addition the higher productivity enabled the use of very small columns that when coupled with a smaller upstream manufacturing footprint, result in a large reduction in the overall size of the manufacturing facility and result in a fully single-use operation.
Some additional benefits of the manufacturing process that were highlighted in closing included:
- Quality and purity of the products purified with the Protein A and other mixed-mode and anion exchange membranes were comparable to reference resin processes.
- Industrial production can now essentially be accomplished at the scale typically seen in pilot facilities, or in some cases, at lab scale.
- Plug and play systems can be deployed for smaller or emerging markets with full operation possible in months.
- One can envision a day when monoclonal antibody cost-of-goods can be reduced even further than the $50/gram calculated here.
After the talk, I was fortunate to be able to talk with Dr. Jacquemart and ask him some questions about his presentation. Here is our interview.
In your presentation, you talk about a holistic approach to improving the downstream process. How important is incorporation of upstream in the process, can you still achieve the same benefits if you are getting upstream from a fed batch process?
Whether your upstream process is continuous (perfusion) or fed-batch, the important consideration is that your downstream process be properly aligned and sized for the upstream output. Traditionally, it was necessary to use oversized resin columns because of their inherently slow operation. This would result in over-investment in what is already very expensive media and equipment. Especially for clinical stage programs, much of that investment usually lost after the campaign due to under-utilization of the media. The new membrane column approach would allow the manufacturer to right-size the downstream to match the upstream and the overall production requirement from pre-clinical through commercial scale. In this way the purification media is fully utilized with every batch, and the end-user optimizes capital use. For a continuous operation, the manufacturer can simply replace the single-use membrane columns during production.
In the past membranes have been seen as being low capacity, how has this changed?
Traditional membranes are inherently low capacity, because their functional chemistry exists only on a limited surface area, which means the target molecules have limited opportunity to be “caught” by the membrane. The first generation membranes were essentially two-dimensional with the active chemistry on the surface. The Natrix HD membranes are sponge-like in their structure, with an infrastructure of large interconnected pores. Every surface throughout the membrane structure is densely comprised of binding sites, so the purification stream is constantly presented with binding opportunities. Depending on the specific application, the result is binding capacity comparable to resins (multiple times greater than traditional membranes), but still at membrane flow rate. This allows high-speed operation of membranes to be combined with the capacity and robustness of resins to create a leap in productivity.
Meanwhile, you also need to think about traditional resin chromatography, which is also more of a three-dimensional operation. However, while traditional resins may have large binding capacity for antibodies, they are characterized by relatively small pore size. This means that binding action relies on diffusion, which results in slow flow rates, large columns, and long cycle times.
Finally, in the context of large molecules (e.g. vaccines production) the binding capacity of resins is actually limited in consequence of the inaccessibility of most of the surface area that is enclosed inside the beads, but not reachable by the large targets.
Single-use has proven its value in upstream. What do you see as unique benefits to a fully single-use operation in downstream?
The big-picture benefits of single-use technology are well-understood: the time and expense of building and operating a cleaning and validation program for the process are virtually eliminated. Instead of having to clean your equipment and validate the cleanliness, you simply discard the depleted device and replace it with a fresh new one. The elimination of cross-contamination risks is also a big advantage. The more nuanced advantages relate specifically to the new opportunities that single-use downstream platforms open-up for the industry. The greatest near-term impact is during clinical development. At any point in time, there are hundreds of pre-clinical programs underway, and in almost every one, the downstream process is relatively fixed while the upstream is highly flexible. This means that upstream processes of all shapes and sizes must be accommodated by something akin to a “one size fits all” downstream approach. This means manufacturers often tie-up millions of dollars in resins and columns and the associated infrastructure, only to leave much of the capacity not utilized. The beauty of single-use in downstream is that a membrane column can be pulled off a shelf to match a given process. After the purification step is complete, the device is discarded and a new one is pulled from the same shelf for the next process. The entire process and infrastructure can be optimized to maximize flexibility, productivity and quality while minimizing total cost. I should add that the single-use approach is a whole lot simpler too!
Are there any other considerations when looking at single-use membrane columns?
When you broaden out beyond the application-specific details, there are some important new avenues that this technology makes possible for the industry. For example, the downstream operation is significantly downsized and does not require the elaborate cleanroom infrastructure that a conventional downstream resin column operation needs. This means a new fully single-use facility is possible, which can be housed in a simple, much-smaller building. The possibility of smaller, less expensive buildings and multi-product operation means that new plants can be located in geographies that previously could not have realistically contemplated mAb manufacturing. Smaller facilities also mean significantly reduced the risks associated with constructing a new plant – facilities would be built much more quickly and at far lower investment and can be modular – expanded as the manufacturing need grows. These newer capital-efficient plants are highly flexible, so they can be rapidly re-deployed for various products. Instead of being a constraint, the new manufacturing facility becomes a nimble tool for the manufacturer. While USP, clarification and polishing operations are already available from various suppliers, the Protein A membrane featured in this presentation is the missing link to create these fully flexible antibody manufacturing facilities.
What is your next project?
Our next project is again with Univercells, and also includes Batavia Biosciences, a CDMO based in the Netherlands and the US. While not all details can be disclosed at this moment, I can mention that the project will use Natrix’s new platform to contribute to a game-changing vaccines manufacturing project that will be funded by a grant from the Bill & Melinda Gates Foundation. The Protein A membrane presented at BPI 2016 is only one example of Natrix’s new affinity membrane platform technology that will also be adapted for vaccine, gene therapy and oncolytic virotherapy processes, and I am looking forward to being able to communicate our progress in the coming years.
