Antibody Fragment Purification Platform
Since the 1980’s monoclonal antibodies have revolutionized medicine and become a vital tool in fighting many diseases. While there are many new monoclonal antibodies in the clinical trial pipeline, there are also some innovative drugs made from just an antibody fragment. Due to the multi-domain structure of antibodies, it is possible to create smaller antibody fragments that still include the antigen-binding domain. Antibody fragments have some advantages over full-length antibodies and several antibody fragment-based biotherapeutics are in clinical research. Several antibody fragments have been approved and are commercially available, including: ReoPro®, Lucentis®, and Cimzia®.
Antibody fragment-based therapeutics also include bi-specific antibodies. Bi-specific antibodies combine two antigen binding regions into a single therapeutic, such as Blincyto®.
Antibody fragments are also being explored for use in antibody drug conjugates where a full length antibody or an antibody fragment is linked to a cytotoxic agent designed to kill target cells when internalized and released; antibody gene therapy applications; and antibody fragments are also being linked with liposomes or nanoparticles to improve drug delivery.
To better understand antibody fragments, their production and purification, my research led me to a very interesting white paper, “A Platform Approach to Purification of Antibody Fragments”, by GE Healthcare. In the white paper, the authors describe a platform purification approach that is comprised of four different resins, each of which provides a platform solution for a specific type of antibody fragment. These resins can be used without the need for lengthy process development or optimization. These four resins together provide a toolbox approach that covers purification of nearly all Fabs fragments and a majority of smaller antibody fragments. In the paper there is a wonderful background on what antibody fragments are, their make up, manufacture and an improved approach to purification.
Antibody Fragments
In the white paper, authors explain that antibody fragments are just that, portions of a full-length antibody. The multi-domain structure of antibodies permits the creation of smaller antibody fragments while still including the antigen binding domain. Antibodies fragments used to be generated by cleaving an intact antibody using an enzyme, but now they are mostly developed using genetic engineering.
Antibody fragments offer some unique advantages as compared with full-length antibodies. The authors describe that due to the smaller size of antibody fragments they can more easily penetrate into tissues. Also due to their small size and because they are non-glycosylated, they can be produced using microbial systems such as E. coli or yeast instead of more costly mammalian cell systems. Lastly, antibody fragments have different pharmacokinetics than full length antibodies and this can be attractive for certain applications.
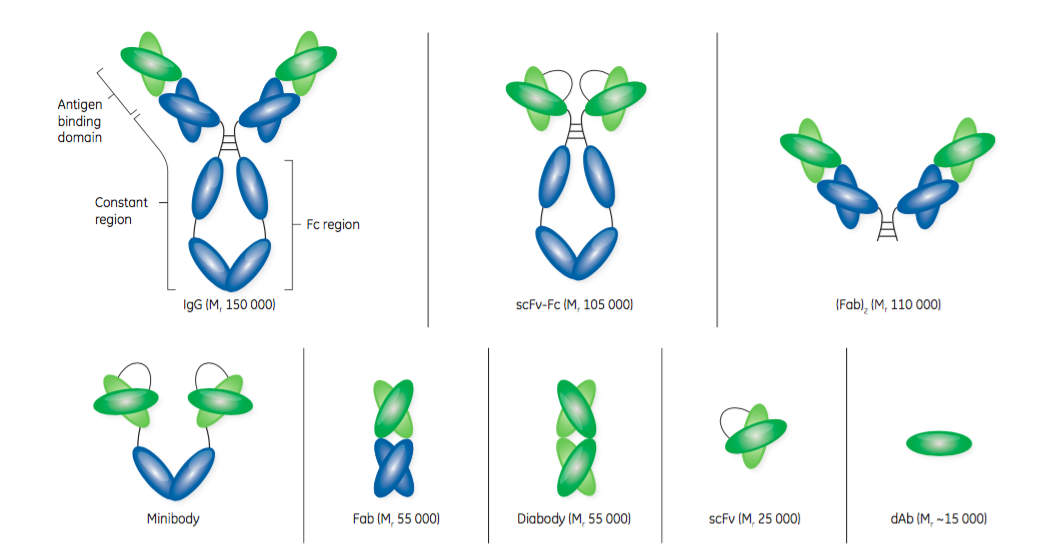
A helpful breakdown of the antibody fragments types was provided in the white paper:
- Fab – “Fabs are considered the first generation of antibody fragments and were initially generated by cleavage of an intact antibody using an enzyme, such as papain. Papain cleavage yields two monovalent Fab fragments, each composed of one variable heavy chain (VH) and one variable light chain (VL) linked by disulfide bonds and displaying a single antigen-binding site. Today, Fabs are produced using modern genetic engineering approaches.”
- “scFvs are monovalent structures, with affinity for a single antigen. With an approximate size of Mr 25 000, an scFv contains the variable regions of an antibody’s heavy and light chains fused into a single polypeptide chain via a short flexible linker. An scFv comprises the complete antigen- binding site of its parental antibody molecule.”
- “dAbs, consisting of the VH or VL domains, are some of the smallest functional antibody fragments that retain full antigen-binding specificity. The dAb is approximately one-tenth of the molecular weight of a normal antibody. Although dAbs contain only three of the six complementary determining regions from the parent antibody, they do exhibit antigen binding specificity and affinity. A dAb can be remarkably stable under harsh conditions of temperature, pressure, and denaturing chemicals.”
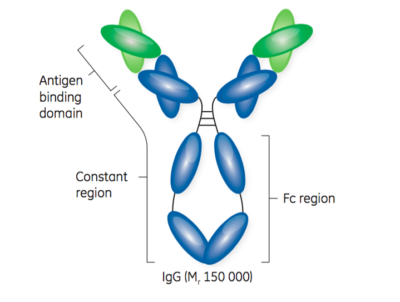
GE Healthcare provides an excellent visual representation of the structure of antibodies and antibody fragments in Figure 1.
Antibody Fragment Purification
One significant challenge in production of antibody fragments is purification. There are a couple main reasons that antibody fragment purification is more complicated. First, production in microbial systems is more difficult when it comes to clarification and primary capture steps. The authors go on to describe the various techniques employed for clarification and purification, but there are limitations for each and thus no method of choice has emerged. Ideally it would be best to employ a platform approach that would allow rapid purification of a wide range of antibody fragments with high product purity and yield. However the non-affinity chromatography options described require process development for each fragment.
Second, purification of antibody fragments is challenging because unlike full-length monoclonal antibodies, there is no FC region. The FC region is a common binding motif, allowing for a platform approach that works with almost all full-length monoclonal antibodies. Purification of full-length monoclonal antibodies commonly includes a Protein A-based capture step followed by one or two polishing steps to remove remaining impurities. Protein A is highly effective as an initial capture step, often providing more than 99% purity with a single step.
Due to the diversity of antibody fragments and the lack of an FC region, development of a specific purification protocol is required for each fragment. The platform approach that has worked so well in monoclonal antibody purification with Protein A has been more difficult to achieve with antibody fragments because of this diversity.
Antibody Fragment Purification – A Platform Approach
The authors then describe GE Healthcare’s platform approach for antibody fragment purification that involves a toolbox of four affinity chromatography resins. These resins can be applied based on the type of fragment begin purified. This approach, the authors explain, enables high selectivity and excellent pressure flow properties for high purity and yield in industrial scale purifications.
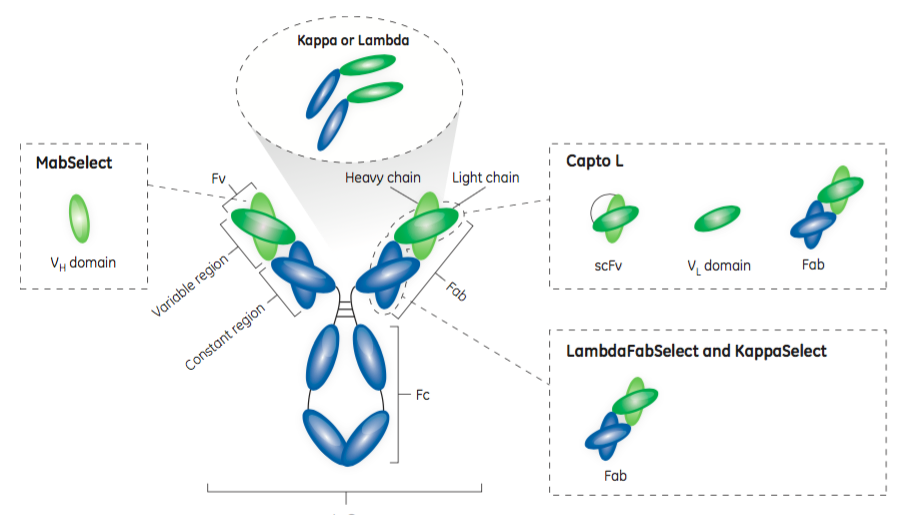
Protein L – Capto™ L Resin
Protein L resins have been available for many years. Protein L targets the kappa light chain, so it can purify fragments derived from antibodies that have the kappa light chain. Because Protein L interacts with the kappa light chain it has no immunoglobulin class restrictions, therefore Protein L can be applied to many antibody fragments, although it is not as widely applicable as say Protein A is with full length monoclonal antibodies. The authors share that “approximately 60% of mammalian IgG light chains are kappa chains.”
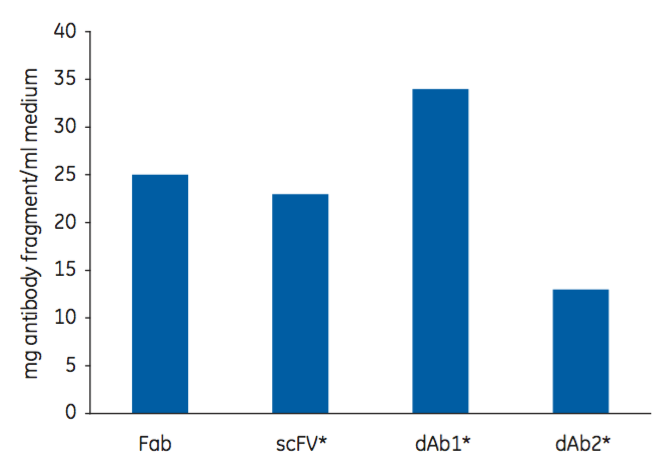
Capto L Resin is one of the four resins in the toolbox. Using a Protein L ligand, it has a broad affinity for a range of antibody fragments that contain kappa light chains. It has been optimized for industrial applications, which permits high flow rates and high productivity as well as low ligand leakage. It is well suited for large scale manufacturing. Figure 2 shows the dynamic binding capacity (DBC) at 10% breakthrough (QB10) of Capto L for four different antibody fragments. Note that, as the DBC is normally measured in mg/mL, the molecular weight of the target molecule is an important factor to consider.
LambdaFabSelect Resin
LambdaFabSelect is another affinity resin in the toolbox. LambdaFabSelect is recommended for the capture of Fabs containing the lambda light chain, approximately 40%. Capto L and LambdaFabSelect can purify nearly all Fab fragments and a majority of the smaller antibody fragments.
KappaSelect Resin
KappaSelect is the third resin in the toolbox and is an affinity resin that binds to the constant region on the kappa light chain. This resin is used to capture Fabs containing the kappa light chain when Capto L is not providing the desired results.
MabSelect™ Resin
MabSelect is a Protein A ligand affinity resin most commonly used in purification of full-length antibodies. While it is often employed in binding the Fc region, it also binds to the VH3 domain subtype of human IgG Fabs. Thus, the MabSelect resin is an important part of the toolbox for antibody fragments, as it can be a useful alternative for capturing heavy chain dAbs that contain the VH3 domain subtype.
In Figure 3, the authors provide an affinity map of the four toolbox affinity resins.
Case Studies
To demonstrate the platform approach for antibody fragment purification, GE Healthcare presented three case studies in the white paper.
Three-step Fab purification process
The first case study was a three-step Fab purification process in which a kappa subclass Fab fragment expressed in E. coli was purified. Capto L resin was used for the initial Fab capture from the supernatant to reduce host cell proteins and endotoxin levels. Then to reduce Fab aggregates, Capto SP ImRes (a high-resolution CIEX resin that allows efficient separation of aggregates from monomers) was run in bind-elute mode as the intermediate purification step. Lastly, Capto Q AIEX was run to remove remaining impurities as a final polishing step. The process developed using a design of experiment approach and HTPD tools resulted in unit operation recoveries >90%. The total process Fab recovery was 87% at an aggregate content of 0.8%. HCP and endotoxins levels were significantly reduced over the process. Protein L ligand leakage was below detectable levels.
Three-step dAb purification process
The second case study was a three-step dAb purification process involving a dAb expressed in the periplasm of E. coli and released by heat treatment of the bacterial suspension. First, clarification was performed using a microfiltration step with hollow fiber filters. Next Capto L resin was run for initial capture and reduction of host cell proteins and endotoxin. For the intermediate purification, Capto MMC ImpRes (a weak CIEX multimodal resin with high selectivity in a broad pH/ salt window) was run in bind-elute mode to further reduce host cell protein. Final polishing was done using the Capto adhere ImpRes multimodal AIEX resin in flow-through mode. Again process conditions were optimized using a design of experiment approach with conditions for optimal dAb purity and yield determined using Monte Carlo simulations. The dAb recovery of the optimized process was 89%.
Scale up of the dAb capture step
The third case study described the scale up of the dAb capture step using ready-to-use products. The dAb capture step was scaled up to pilot manufacturing by using a 2.5 L prepacked ReadyToProcess™ Capto L column and ready-made HyCloneTM buffers. ReadyCircuitTM bags and tubing assemblies were used to enable closed system operations. Custom made cGMP manufactured HyClone buffers were delivered in single-use containers that could be directly connected to the equipment. Reproducible results from triplicate runs indicate process robustness. Results from the scaled-up process were comparable with those from the process run at laboratory scale.
Capture of scFv fusion protein from a challenging feedstock
The last case study was presented on the capture of a human scFv fusion protein from a challenging feedstock. Capto L resin was used for capture of scFv from animal plasma because of its high selectivity and high capacity. By optimizing process conditions, with regard to wash and elusion pH, a scFv recovery of 93% at high purity could be achieved in the developed capture step.
For more information and full data about utilizing a platform approach to antibody fragment purification, and to view the full white paper, please see – A Platform Approach to Purification of Antibody Fragments.
