Combining Ion Exchange and Reverse Phase Chromatography for Highly Efficient and Cost Effective Peptide Purification
Therapeutic peptide purification can be very challenging because of the similarities between peptides and their impurities. There is not a significant difference in retention for the target peptide and a peptide contaminant that has one amino acid that is different. Thus, separation of peptides from by-products requires long retention times that are inefficient. In addition, even small changes in the mobile phase composition can cause significant effects. One way to increase the separating power of RP-HPLC is to make use of an ion-pairing agent, which couples with basic amino acids, resulting in a net zero charge. Generating a net zero charge status enables separation using a reverse phase process. However, the duel reverse phase process can be cost prohibitive for peptides that require a high level of purity.
To address the challenges of therapeutic peptide purification, Purolite worked with collaborators to create a novel process that combined ion exchange chromatography with reverse phase. This novel process was used in a case study and the results are described in a recent webinar, “Case Study- A novel combined IEX-RP chromatographic process for the purification of peptides”. Highlights of the webinar are included in this article.
Development of a Novel Chromatographic Process
Presenter, Dr Benjamin Summers, Purolite Applications Specialist, began by explaining why ion exchange chromatography is generally avoided in peptide purification. This is due to the difficulty in removing both polar and non-peptide impurities and peptide by-products. However, he goes on to say that a highly hydrophobic cation ion exchange stationary phase offers the possibility to carry out the ion-pairing process in reverse. This approach also facilitates the removal of upstream impurities, such as polar non-peptide impurities, and the possibility for virus clearance due to the low pH of the process.
Case Study – Bivalirudin Purification
Dr. Summers described a project with Purolite and Novitide and the resulting case study to purify the peptide bivalirudin. Bivalirudin is a thrombin inhibitor, an alternative to heparin as a blood thinner with significantly reduced side-effects. Since it is dosed via IV, a very high level of purity is required.
Bivalirudin is a relatively long peptide with a molecular weight of 2.2 kDa and an isoelectric point of 3.9 (Figure 1). In order to achieve binding to a cation exchange resin, they needed to use a relatively low pH of 2.5. They used a modified resin, PCG1200FS, with strong acid cation exchange groups, which permitted use of such a low pH.
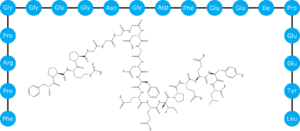
The ion exchange modified, highly hydrophobic resin Chromalite® PCG1200FS was used for the first step. Then reverse phase chromatography provided the final polishing. Details of both resins used are in Figure 2.
Chromalite® Resins Combination for Peptide Purification

Important to note, the particle size of PCG1200FS is much larger than reverse phase. The increased particle size makes the process financially viable. Using a larger resin for the first step reduces the cost of peptide purification.
Ion Exchange Purification Step
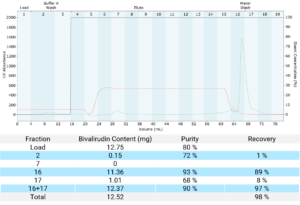
For the ion exchange step, the bivalirudin crude sample was loaded in 20 mM citrate at a pH of 2.5. The affinity of the bivalirudin for the combined hydrophobic attraction and ion exchange resulted in elution in water and wash after removal of impurities. Pooling fractions 16 and 17 resulted in a bivalirudin fraction that went from 80% pure sample to greater than 90% pure with greater than 90% recovery (Figure 3).
Ion Exchange Purification
Reverse Phase Polishing Step
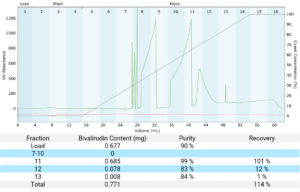
For the reverse phase polishing step the sample was loaded with combined fractions 16 and 17 followed by wash and elution. The reduced particle size compared to initial cleanup resulted in increased resolution of peaks. The high resolution reduced peak volume, thus simplifying further cleanup (Figure 4).
Reverse Phase Polishing
Results
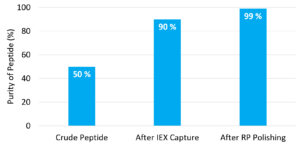
With the positive results seen in the case study, study authors believe that the combined ion exchange and reverse phase approach would permit starting with 50% pure crude peptide, then utilizing the IEX capture step to achieve greater than 90% purity. Finally followed with a reverse phase polishing step purity of 99% can be achieved (Figure 5).
Combined IEX-RP Approach
Conclusions
The combination of using a low pressure ion exchange step (50-100 micron) with standard reverse phase (12-18 micron) polishing was demonstrated using Chromalite® PCG1200FS and Chromalite® 15AD2. This resulted in a peptide purification process that was more cost-effective than the traditional two-step reverse phase process. The is because the low pressure ion exchange step is much more cost effective than the reverse phase step. This novel process permitted bivalirudin purification from a semi-crude feed of 80% purity to greater than 99% purity with greater than 90% recovery.
For more information, please view this and other webinars in Purolite’s Digital Learning Series.
