Aramus Single Use Bag Assembly – Provides Advantages over Existing Storage and Transportation of Intermediate and Drug Substance
Safe and efficient transportation of drug substance from one location to another requires considerable time and resources. With the rise in the use of Contract Manufacturing Organizations (CMOs) and Contract Fill/Finish providers, there is an increasing need for efficient tools to enable these processes and an increase in the logistics required.
Stainless steel cans are currently most often used for freezing and shipping of drug substance. However, there are challenges with using these containers. First, the shipping costs are higher because of the weight of the cans and since they are reusable, they need to be returned. They also need cleaning and validation, both prior to use, and upon return. Lastly they require a fair amount of capital investment up front and maintenance cost ongoing.
The expanded application of single use bags across biomanufacturing processes has resulted in examination of single use bags for drug substance transporation. Single use bags provide an attractive option for bulk freezing and shipping. The advantages include lower shipping cost as bags weigh less and don’t need to be returned. There is no need for cleaning or validation and they require less capital investment and maintenance cost. However, there are concerns about using single-use bags due to possible leachables/extractables, particularly with high-value final products. Bag integrity is also a concern as breakage or leaks would result in contamination and would be very costly.
Because there are a variety of different bags designed for different purposes, end users have to ensure that they are selecting the best bag for their particular application. They must also evaluate the bags and determine the benefits and risk associated with using a single use bag vs. a stainless steel can or other reusable option.
Specially Designed Single Use Bags
To address these challenges, Entegris has launched a line of Aramus™ Single Use 2D Bag Assemblies. These bags are made of a high-grade, gamma-stable fluoropolymer, providing higher purity, greater compatibility and increased safety for critical process fluids and final products. Entegris describes that their new single-layer technology contains no curing agents, antioxidants, plasticizers or adhesives, so the number of potential contaminants is greatly reduced.
Other advantages include a wide operating temperature range for frozen applications (to -85°C [-121° F] or lower) without compromising the integrity of the bag. Because the bags are single layer, the extreme freezing temperatures do not cause delamination either.
Entegris demonstrated this durability in low temperatures in a recent video:
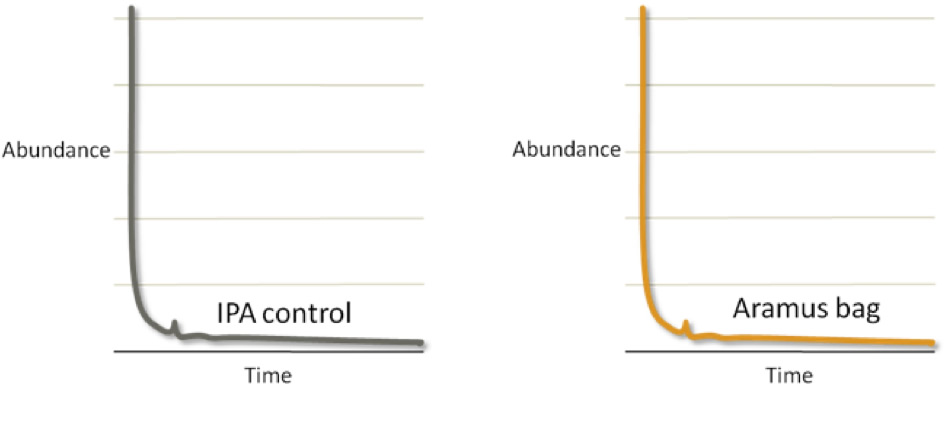
To test for extractables, Entegris exposed process contact surfaces to 100% IPA for two weeks at room temperature and analyzed by GC-MS for organic compounds. The result was that Aramus bag assemblies showed no extractable organics. (Figure 1)
Figure 1: Extractables testing
While the focus of this article has been on final fill applications, Entegris offers a number of applications for the Aramus single use bag assemblies including:
- Frozen product storage
- Downstream bioprocessing
- Process Sampling and Archiving
Current sizes include 500 ml, 1 liter and 2 liter with 5L, 10L and 20L bags coming next year.
For more information, please see – www.entegrislifesciences.com/aramus or lifesciences@entegris.com.