Residual Enzyme Testing – All in one Assay Kits for Liberase and Trypsin
Background
Enzymes are a very necessary part of biomanufacturing for both traditional biopharma and cell therapy applications. However it is important that these enzymes be removed prior to delivery of the final product. Residual enzymes can potentially cause immunogenic activity, reduced efficacy of the final product and even toxicity in patients. If not removed sufficiently, they can result in product contamination. As a result, companies need to validate the effectiveness of their purification processes to ensure sufficient enzyme removal. In some instances, regulatory agencies will advise companies to consider testing for the presence of residual enzymes as part of an overall risk-based assessment for each biopharmaceutical or cell therapy product.
Key Issues
Considering the possible negative impact residual enzymes can have, ensuring removal is very important. However it is not just sufficient to remove the enzymes. Companies should be able to verify and document that these residual enzymes are in fact no longer present in the final product. When verifying removal of residual enzymes there are some key issues to consider:
- What are the regulatory requirements on residual enzyme removal for your product and is your current process meeting those requirements? If so, how will you validate the removal and will it be sufficient to meet regulatory requirements? Are your assays acceptably sensitive, specific, and consistent?
- What impact could the presence of residual enzymes have on your final product performance or on patient safety? Can you detect the presence of inactive protein fragments in addition to the active enzyme?
Testing Methods
When testing for residual enzymes there are a few options that most companies employ. First they either decide to test in-house or send the samples out for testing. Sending samples out for testing saves time in-house, however it can create delays and a more complicated workflow. When testing in-house, a company maintains control over samples and can plan to test when and as needed, however these tests can be time consuming and require sourcing of all necessary testing materials. When testing in-house, companies must decide what testing methods they will use.
Some groups create their own assay kits and this can sometimes represent a more affordable testing option. However the challenge with creating an in-lab kit is that the company must obtain testing components from multiple sources, which can be time consuming. Most often, these components are not pre-validated, requiring validation to be done in-house which is also time consuming. Additionally there is the risk of variability from assay to assay based on variability of sourced components.
Another option is to purchase all-inclusive kits that are ready to use. While this option might be slightly more costly up front, these commercial kits do provide significant time savings and a reduction in potential variability of assay results.
All-inclusive ELISA Kits for Residual Liberase and Trypsin Testing
Earlier this year, Roche Custom Biotech launched two new products, the Residual Protein Liberase Kit and the Residual Protein Trypsin Kit, to address some of the key challenges associated with current residual enzyme testing methods.
Sensitivity and Specificity
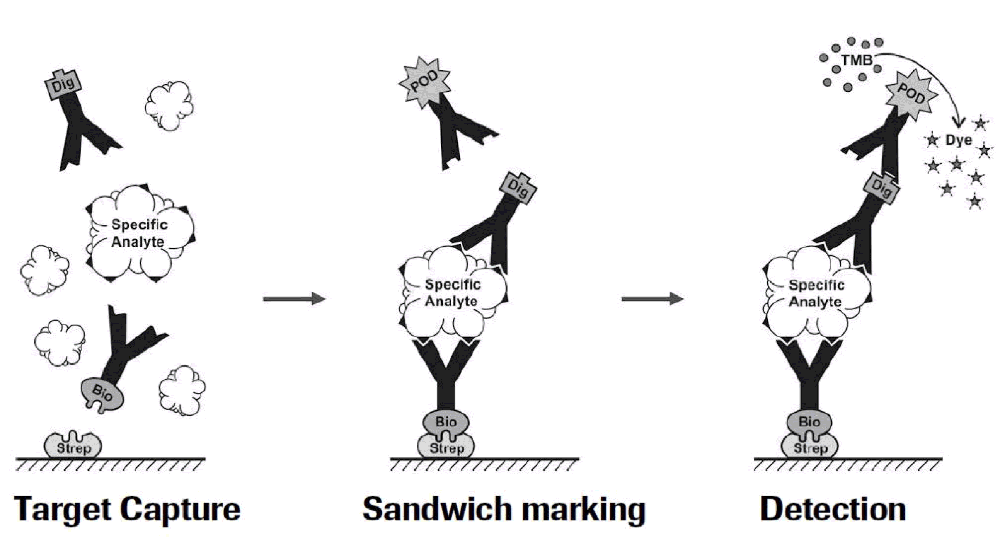
Currently, many assays have limited sensitivity and specificity in that they only detect active enzymes and not inactive protein fragments that might still be immunogenic. With the Residual Protein Liberase Kit, Roche uses ELISA technology with polyclonal antibodies specific for collagenase I, II and thermolysin (enzymes contained in the Liberase blends). Similarly, the Residual Protein Trypsin Kit uses polyclonal antibodies specific to Roche recombinant porcine trypsin. These tests are independent of enzyme activity and will detect the proteases and also fragments thereof with very high sensitivity. The limit of detection for residual Liberase is 0.1 ng/ml and for residual trypsin is 0.5 ng/ml.
Reliability and Consistent Results
Reagents contained in these kits offer high lot-to-lot consistency. They are also validated and have been tested together for the intended applications, detection of residual Liberase or Trypsin enzymes. Additionally, the kits are manufactured in an ISO 13485 certified facility. These features provide more accurate and consistent assay results overall.
Simplified Workflow, Ease of Use and Faster Results
Included in each kit is a pre-coated 96-well plate with 8 well strips for enhanced convenience and flexibility. Also included are standards and controls for ease of use. The kits also offer a fast turnaround time with results in ~2– 2.5 hours. Finally, because the kits are ready to use there is no need for specialized employee training.
Combining these kits with Roche animal-free, GMP-grade enzymes augments production efficiency, from the use of high-activity enzymes to timely verification of their clearance. For more product information, please visit Custombiotech.com/testkits.
Figure 1: Residual Liberase and Residual Trypsin Kit Testing Workflow:
Roche Custom Biotech contact information:
Tel: +1 800 428 5433 ext. 14649 (toll-free)
Email: custombiotech.ussales@roche.com