Bioburden Contamination in Downstream Bioprocesses – Potential entry points for contamination and innovative solutions
Bioburden contamination in biopharmaceutical manufacturing is a big concern. Contamination carries both tremendous cost and preventing it requires strict control of several possible entry points. The cost of bioburden contamination for a company can involve lost time, lost material, batch loss, possible facility closure and extensive QA/QC time to ensure proper cleaning and validation. In the worst case scenario, it can prevent supply of much needed medicine to patients and loss of commercial revenue.
It is a challenge to manage because there are several possible entry points for contamination in both upstream and downstream bioprocessing. Upstream offers a unique set of challenges because you have media and culture systems specifically designed to encourage growth. However that growth friendly environment exists not just for cells but also for bacteria and viruses. In addition, in upstream you are introducing media, raw materials, personnel and even the cells themselves that can all introduce contamination.
Downstream bioprocess also has its share of entry points and contamination risk. After writing several pieces which included the possible introduction of contaminants in upstream, I decided it was time to examine the introduction of contaminants in downstream, possible entry points, and some innovative solutions to address risk.
Points of Introduction
In downstream operations, there are several activities that present risk either through materials introduced or the open system in which they are conducted.
Entry points for downstream:
- Protein A capture step – combines cell culture nutrients with Protein A resin that can be sensitive to harsh cleaning.
- Column packing – a manual, open operation that can be quite messy and often involves the transfer of equipment between rooms.
- Buffer preparation – manual, open operation that involves large volumes of fluid, and large tanks that can be difficult to fully sanitize.
- Addition of any materials to the process – solutions, water, airflow
- Surfaces – the introduction of liquid to any surface that could be contaminated including interfaces between units, probes, filters, columns, chromatography, etc.
- Facility – movement of equipment or personnel around the facility and improper maintenance or sterilization.
I found an interesting infographic by GE Healthcare that provides a nice visual of the overview of both bioburden entry points and key areas to consider as areas to reduce risk.
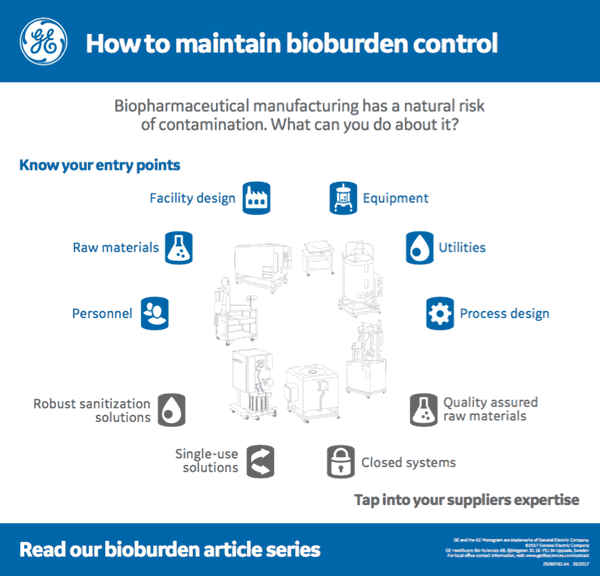
While every company will have Standard operating procedures (SOPs) and appropriate monitoring in place to reduce the risk of bioburden contamination there are also some manufacturing strategies that can further reduce risk.
Manufacturing Strategies
Certain manufacturing strategies can be implemented to reduce the opportunity for contamination. First, disposable products have been widely adopted in smaller scale operations and are also being explored at larger scale. While being completely disposable may never be feasible for large-scale manufacturing, implementing this approach in areas where possible does reduce the cleaning and sterilization requirement. Next, closed systems are growing in availability and implementing this kind of system reduces the number of entry points for bioburden. Lastly automation, which often pairs with closed system operations, is becoming more widespread, thus reducing the amount of manual operations. Reducing manual operations limits involvement of personnel and the inadvertent introduction of contaminants through human error or handling.
Innovative Solutions
One interesting resource I found in my research on reduction of bioburden risk and possible solutions was a webinar by GE Healthcare called “Setting New Standards for Sanitization of Chromatography Resins – Addressing bioburden challenges in downstream bioprocess.” The talk, presented by Dr. Günter Jagschies, Senior Director, Strategic Customer Relations and Anna Grönberg, M.Sc. Staff Research Engineer, began with a discussion of the cost of bioburden contamination. It continued by addressing ways of preventing bioburden introduction in downstream. Followed by a case study of sporicidal sanitation of Protein A affinity resin and a discussion of products and prototypes that could provide more robust solutions for reducing bioburden risk.
Dr. Jagschies discussed the various points of entry and also ways gain more control over these entry points.
Sources of entry to control:
- Suppliers and biopharma must collaborate to minimize bioburden
- Design closed systems
- Supply quality assured raw material
- Introduce single-use solutions
- Enable robust sanitation process
Robust Sanitation Process
Grönberg went into further detail on robust sanitation processes. She began by explaining that traditional bacterial contaminants are more successfully eliminated by using a 0.5 M NaOH solution for CIP and sanitization over 0.1 M NaOH.
Table 1: 0.5 M NaOH effectively inactivates bacteria, mold and yeast
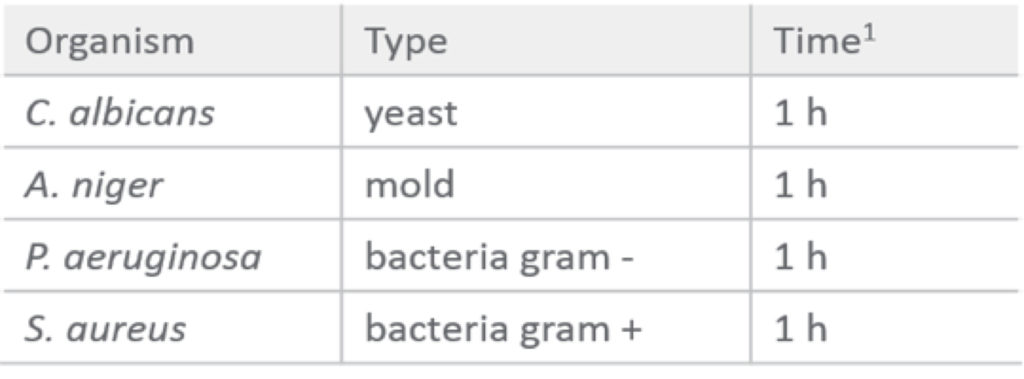
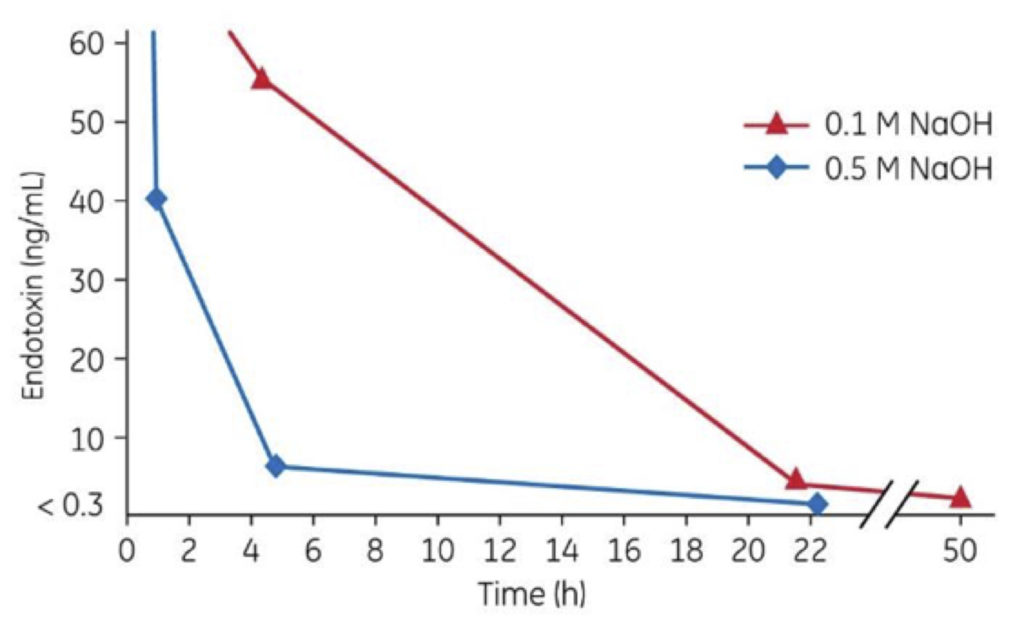
Figure 2: More effective endotoxin inactivation with increasing NaOH concentration
She went on to explain that while 0.5 M NaOH is effective with most bacteria, bacterial endospores are not effectively inactivated even with high concentration and extended contact time (Figure 3). Solvents are very effective at inactivating these spores, but they are not compatible with large scale biomanufacturing or protein based resins.
Figure 3: Reduction of B. subtilis spores in 50% MabSelect SuRe Slurry
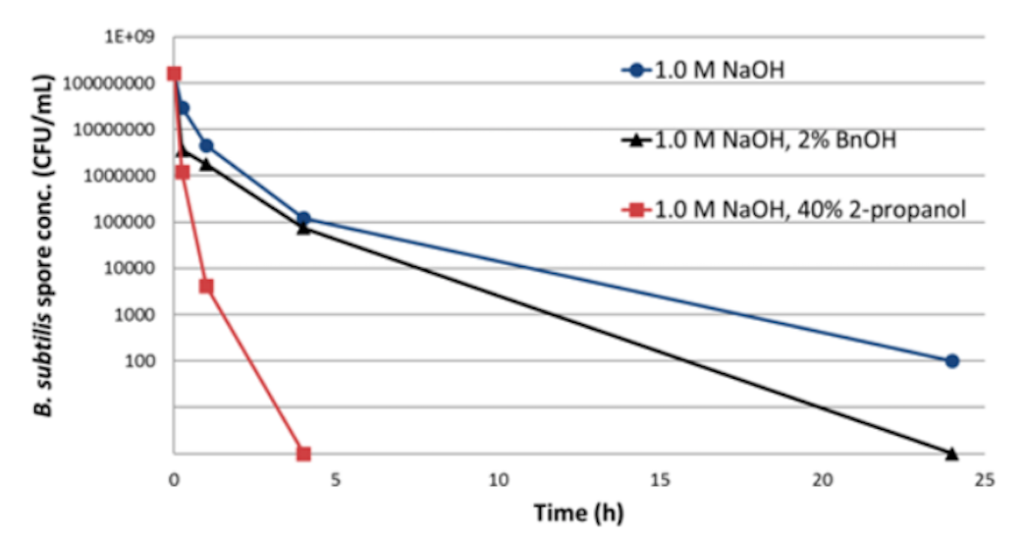
Because these spores are so difficult to inactivate using standard methods, it becomes even more critical to reduce the introduction of bioburden contaminants into the downstream process.
Sporicidal Sanitization of Protein A affinity resin
Another solution to the challenge of inactivation of bacterial spores is the use of a more effective method. Peracetic acid (PAA) has been proven to be more effective in the inactivation of bacterial spores. Grönberg presented compelling evidence about the effectiveness of this method (Figure 4).
Figure 4: Reduction of B. Subtillis spores in 50% MabSelect SuRe™ Slurry
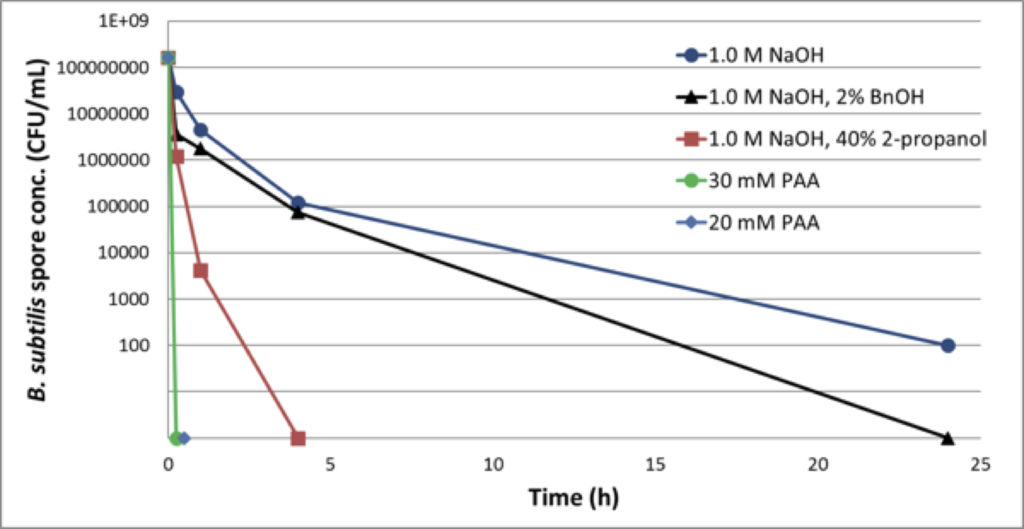
Despite the obvious success, PAA must be compatible with affinity resins. GE Healthcare studied the effect of PAA on high-flow agarose base matrix properties and found no significant effect after treatment with 30mM PAA for 24 hours.
MabSelect SuRe was subjected to 20 mM PAA in a total of 28 treatments with a total contact time of 14 h over 108 protein A process cycles. Although an additional capacity loss of 10% after 50 cycles, compared with the untreated resin, no major negative impact of PAA treatment was observed on mAb recovery and HCP reduction. Considering the big impact that a bacterial contamination incident might have on biomanufacturing, the results show that PAA could still be a viable option for use in industrial process decontamination without major impact on mAb purification performance in the capture step using MabSelect SuRe resin.
PAA is a strong oxidizing agent which might be expected to have negative effects on the performance of chromatography resins, in particular those containing protein ligands. However, studies show that limited contact with PAA (2 to 3 times during the lifetime of MabSelect SuRe) can be used to provide a good safeguard against spore-forming bacteria, without having an unacceptable impact on the resin performance and lifetime¹. However, frequency of PAA treatment should be determined case by case, and column lifetime should be evaluated for each individual mAb process.

Conclusions
While bioburden contamination remains a major issue in biopharmaceutical manufacturing there are strategies available that can reduce the risk of introduction. There are also products and technologies in development that may reduce the risk further and/or enable more robust sanitization processes to improve sanitization in the future.
GE Healthcare has a number of articles on bioburden contamination, please see the entire offering at Bioburden sources you might have missed
¹Application note: Impact of sporicidal agent on MabSelect SuRe™ protein A resin lifetime. GE Healthcare, 29262168, Edition AA (2017)
Previous Related Downstream Column Articles:
- https://downstreamcolumn.com/cost-impact-bioburden-incident/
- https://downstreamcolumn.com/addressing-buffer-bottlenecks-using-automated-line-conditioning-ready-made-stock-solutions-mab-processes/
