
Continuous Downstream Processing – A Tool to Address Key Manufacturing Challenges
Background
Continuous processing is a very successful model in many other industries. Recently, the biotech industry has begun investigating this model in more depth and has started to incorporate certain aspects of continuous processing into biopharmaceutical manufacturing. One area where it can be particularly helpful is in addressing rising titers and improving facility fit challenges.
A decade or more ago, companies were very focused on how to increase mAb titers, but that has shifted significantly as titer levels have steadily increased in upstream culture. Now downstream is facing challenges presented by rising titers and facilities that were designed for traditional batch processing and titers in the range of 5 g/L or less. Continuous processing has been shown to effectively increase productivity and facility utilization in several instances. Some reasons to consider a continuous process or semi-continuous option include addressing facility fit issues and improving facility flexibility and utilization. Product quality concerns, particularly for unstable products where delays in processing could negatively affect quality, can also be managed more effectively with continuous processing.
Real-World Example
A recent webinar excellently described these manufacturing challenges and valuable continuous processing solutions in downstream. The webinar, the Future of Continuous Downstream Processing, A Conversation with Biogen Idec and GE Healthcare Life Sciences, hosted by International BioPharm described the real challenges faced by Biogen Idec as their titers have increased and facility fit has been tested. It also covered the continuous processing solutions tested by Biogen Idec and how GE Healthcare Life Sciences has approached the area of continuous downstream processing.
The webinar began with Sanchayita Ghose, Principal Engineer/Associate Director of Biogen Idec, describing some of the manufacturing challenges they were facing and how this led them to consider continuous processing. Their primary challenge was that increasing product demands required new innovations so that existing facilities could still be utilized. Then Roger Nordberg, Senior Product Manager, GE Healthcare Life Sciences discussed some of the innovative continuous downstream tools designed to solve these types of problems. Lastly Annika Forss, Senior Research Engineer, GE Healthcare Life Sciences walks through a case study to evaluate continuous processes versus batch processes in downstream.
Dr. Ghose began by sharing that Biogen’s Idec’s upstream group had been successful in increasing titers to keep up with product demand. She said that titers at “6-8 g/L is the current reality,” but this increase in titer created new challenges, specifically downstream bottlenecks and facility fit issues. As a result of these changes and because they didn’t want downstream to limit throughput, Biogen Idec began looking at continuous processing as a way to achieve productivity improvement goals.
Biogen Idec already had success with implementing one continuous processing tool. They had been able to decrease culture time in bioreactor by 20-30% by using an N-1 perfusion seed culture in upstream. They began to consider the use of continuous processing in downstream. Dr. Ghose stressed that they were not looking to move to complete continuous processing at the moment, but rather they were looking to implement continuous processing in key areas to address specific needs.
Their first step, she said, was to consider what was causing the downstream bottlenecks. First, there was a significant problem with facility fit as these legacy facilities were built for lower titers, 5 g/L or less. Increasing titer caused challenges with lower throughput of the Protein A capture step and rising intermediate pool volumes. The process intermediates volumes started to exceed their tank capacity of 6,000-8,000 liters. To solve this problem using a traditional approach would mean tank replacement and with that would come cost, validation and facility shutdown.
They knew that it was possible to address these challenges by eliminating the intermediate product hold tanks, but they needed an engineering partner to implement the strategy and were interested in GE Healthcare’s Straight Through Processing (STP) system.
Once they had a plan, they set out to conduct proof of concept studies, but they quickly ran into their first issue – these tools were available in large scale, but no off the shelf scale down system existed, so proof of concept studies at small scale were going to be impossible. They worked with GE Healthcare to reconfigure an AKTA pure system to achieve most of the functionality of an STP system at small scale. The lab scale results showed very comparable product quality and productivity with batch process and they were able to eliminate the need for product hold tanks thus providing a solution to their facility fit issues.
Dr. Ghose closed by adding that interest in continuous or semi-continuous processes are growing and believes that there might be a paradigm shift in the biopharmaceutical industry in the next decade.
Downstream Continuous Processing Tools
Next Roger Nordberg walked through the details of downstream continuous process. He explained recognizing the need for continuous processing and details about transforming batch processes to continuous. He shared that when looking at changing batch processing to semi-continuous processing, it is important to remember that batch processing isn’t designed to be continuous. Batch processing contains too many steps, relies on offline analysis, no dynamic control and most support processes are also in batch mode. In batch processing, capital expenditures and operating expenses are higher; there is also a set footprint that limits manufacturing flexibility in capacity, lead time and facility utilization. In considering whether your process can convert, it is important to remember that fewer steps are easier to convert, automation is key to securing quality, and the change should result in an increase in productivity.
Overall benefits of continuous processing include:
- Reduce cost by utilizing system and media to full potential
- Connect from upstream perfusion to capture and the entire downstream process
- Capacity at all scales reduces the size of your equipment
- Avoids stability issues with the product and shortens the time from start to finish
Mr. Nordberg then discussed the concept of periodic counter current (PCC) chromatography and specifically the AKTA pcc technology platform.
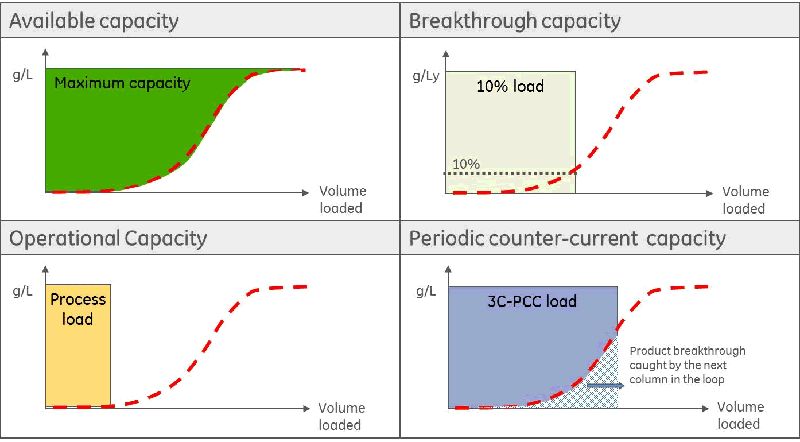
One benefit of using PCC Chromatography is that you can utilize the full potential of the chromatography media by employing a three-column operation. He explained how you begin with Column A and you load it to 60-80% breakthrough, then you disconnect that column and follow with wash, elution and equilibration. While that is happening you switch to Column B and then load that to breakthrough and follow the same steps switching to Column C, etc. By the time you get to Column C, Column A is ready to come back online and you can repeat the process.
One key question is – What happens if the breakthrough curve changes?
The answer is that reliable dynamic control secures the feed. Dynamic control:
- Automatically accounts for variation in feed concentration and/or chromatography medium lifetime effects
- Enables operation at optimum column loads
- Can improve chromatography medium lifetime by limiting exposure of the medium to feed depleted from the product.
Annika Forss builds on Mr. Nordberg’s talk by presenting a case study that GE Healthcare Life Sciences conducted on downstream mAb production using a continuous process. She has also co-authored a technical brief on this topic titled “Toward a unified process development strategy for batch and continuous chromatography.”
She does a wonderful job describing the case study in detail, which I won’t recount here. You can learn the specific details by viewing the webinar, but I have provided some highlights below.
Highlights from the Case Study:
Protein A was used for initial capture due to its robustness and high purity in the elution pools. Remaining impurities were removed using 1 or 2 polishing steps – cation exchange or multi-modal chromatography.
Capture Step:
10 Cycles were conducted using three-column Periodic Counter Current on AKTA pcc with MabSelect SuRe columns loaded to 50% breakthrough using dynamic control
Results:
- Increased capacity utilization by 56%
- Robust performance over 10 cycles with minimal variation in mAb amount, aggregate content and host cell protein content between columns.
- Confirmed dynamic control by using different columns. Column 1 was MabSelect SuRe and column 2 and 3 were MabSelect SuRe LX Media (higher capacity). As a result the system detected the lower capacity on column 1 and put the column on hold with a lower mAb amount eluted from column 1 compared with 2 and 3. This demonstrated that the system adapts run to performance of individual columns.
Purification was performed using straight through processing (STP) defined as “two or more chromatography steps connected in series, in-line adjustment of process streams between columns to ensure right conditions for the next step.” She then described important items to consider when using STP and the development of a STP.
Key steps identified in the development of STP include:
- Individual studies on the different chromatography media.
- Conditioning study based on results of media study.
- Verification runs.
Results: “A STP step with 2 columns connected with in-line adjustment in-between was developed. The results showed similar yield and purity as batch runs.”
For more information:
- Webinar – Future of Continuous Downstream Processing,
- Technical Bulletin – “Toward a unified process development strategy for batch and continuous chromatography.”
