Novel Affinity Resins Enabling the Purification of Next Generation Antibody Fragments: KANEKA KanCap™ G and KanCap™ L
Ever since the licensing of first monoclonal antibody (mAb) drug, biologics have seen unprecedented growth as drugs to treat a variety of malignancies1. The modular structure of mAbs have allowed protein engineers to create smaller, nimbler, and multispecific next-generation antibody therapeutics. Some of these modalities can be easily produced using microbial expression systems, offering higher yields and excellent process economy2.
Smaller sized next-generation modalities can have easy access to difficult targets and often elicit reduced immunogenicity. Additionally, they have been shown to lack bystander effect and makes it possible to target multiple epitopes3. Fabs, diabodies (dAb), single chain Fragment variable (scFv), bispecific scFv (Bis-scFv), ScFv-Fab, Fc modified full IgG, Dual-affinity Re-targeting Antibody (DART) are some popular examples of next-generation antibodies3. However, the purification of the next generation modalities that lack or have modified Fc domain is not straightforward since it precludes efficient binding to most existing protein A resins.
To address this need, Kaneka has developed two cellulose-based affinity resins: KANEKA KanCap™ G (KanCap™ G) and KANEKA KanCap™ L (KanCap™ L). KanCap™ G is developed using modified Protein G ligand and KanCap™ L consists of our proprietary Protein L ligand.
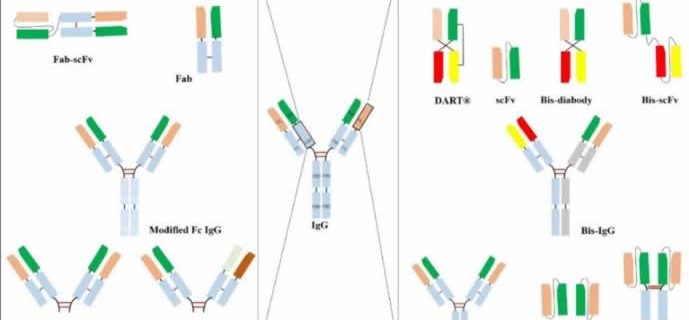
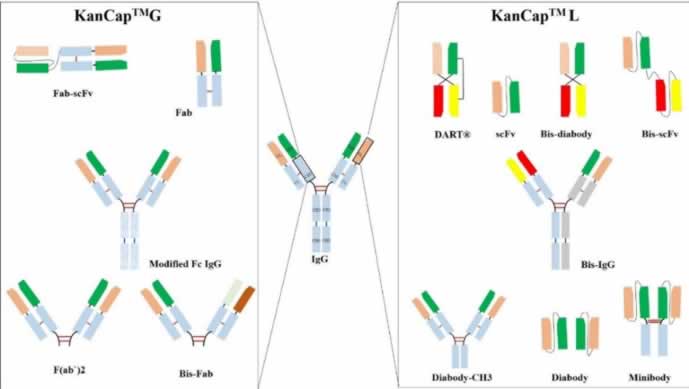
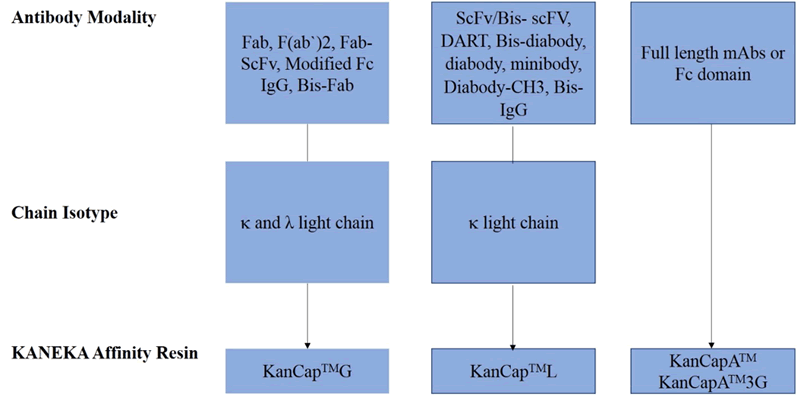
Specifically, KanCap™ G binds to the CH1 domain of Fabs and thus an excellent resource to purify all types of human Fab (k and l type). Whereas, KanCap™ L binds to the VL domain (only for k types) of the antibody and therefore geared towards the purification of Fab, scFv, and diabody like modalities. Figure 1, provides a general description of antibody fragments that can be purified by KanCap™ G and KanCap™ L.
KANEKA KanCap™ G
KanCap™ G is a newly developed cellulose-based affinity resin for the purification of Fab, F(ab’)2, Bis-Fab, Fab-scFv etc. Its proprietary Protein G ligand exhibits an increased binding affinity to the CH1 domain, thereby making KanCap™ G a powerful tool for efficient capture and purification of antibody formats containing CH1 domain. It can also be used as an alternative tool for full length antibody purification.
Performance
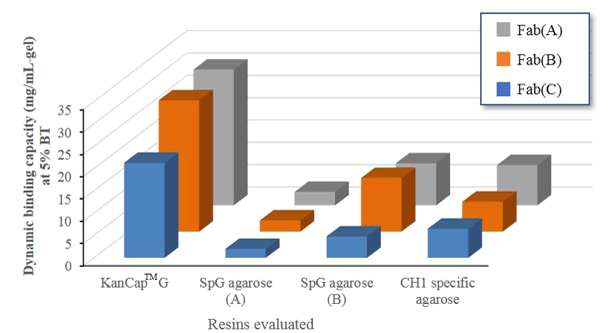
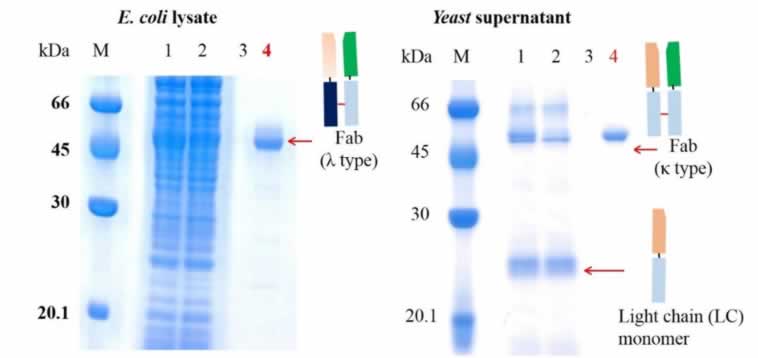
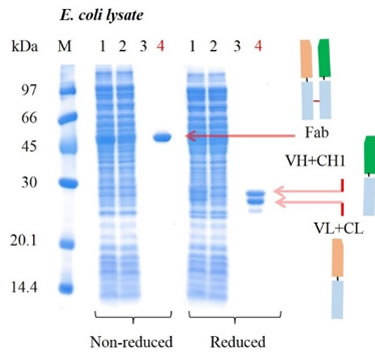
To test the comparative performance of KanCap™ G, three Fab molecules (k and l type) were purified using KanCap™ G and existing products in the market. Our data shows that KanCap™ G offers best in class dynamic binding capacity and outperforms existing protein G resins (Figure 2). It is noteworthy that purification of different Fab modalities using KanCap™ G also results in high level of protein purity as adjudged by SDS-PAGE (Figure 3)
KANEKA KanCap™ L
KANEKA KanCap™ L is a novel cellulose-based Protein L resin that exhibits an increased affinity to VL region of antibody κ light chain. This resin is designed for capture and purification of antibody fragments such as Fab, scFv, diabody as well as an alternative tool to purify full-length mAbs containing a κ light chain that poorly binds to Protein A resins.
Performance
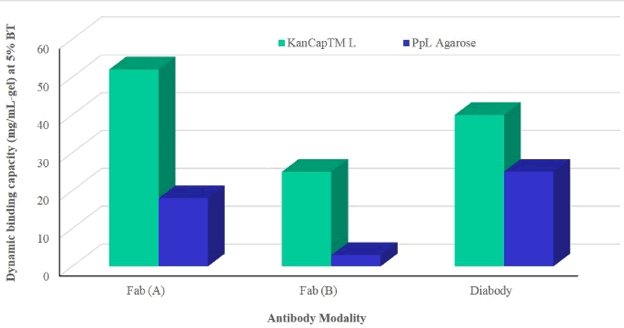
KanCap™ L’s performance was compared to existing protein L resin by purifying two Fab modalities and one diabody. KanCap™ L also offers best in class binding capacity and outperforms competitor’s agarose-based protein L resin (PpL) (Figure 4 & 5).
Summary
The rapid development of mAbs have benefited from the existence of protein A based platform resins. However, the development and purification of next-generation antibodies lacking Fc domain is complex. Kaneka’s KanCap™ G and KanCap™ L affinity resins address this challenge. As shown above, KanCap™ G exhibits higher binding capacity and aids in the recovery of all types of human Fabs (k and l type). Whereas KanCap™ L offers wide binding spectra and excellent binding capacity for k light chain containing antibody modalities.
In summary, to address the downstream processing challenges, KANEKA has developed a robust toolset of cellulose-based affinity resins. As shown below, our protein A resins (KanCapA™ and KanCapA™ 3G) are designed to bind molecules containing Fc domain. The unique features of this class of resins include high dynamic binding capacity, milder pH elution, and excellent ability to resolve impurity contents (aggregates, HCP, DNA etc). These resins can be used to purify full-length mAbs and modalities that have Fc domain.
Our KanCap™ G and KanCap™ L fulfill a critical gap in addressing the purification challenges associated with the discovery and development of next-generation antibody modalities of significant medical importance.
For more information, please visit: http://www.bioseparation.kaneka.com/
References
- Ecker, D.M.; Jones, S.D.; Levine, H.L. MAbs 2015, 7
- Fernandes, J.C. Drug Discovery. Today 2018, 23, 1996–2002
- Bates A, Power CA. Antibodies (Basel). 2019 Apr 9;8(2). pii: E28. doi: 10.3390/antib80200
