Protein A – Evolution and Optimization Strategies
As biopharmaceutical manufacturing has continued to evolve, so have the technologies that support the industry. The use of Protein A has become such an institution in monoclonal antibody purification that I hadn’t given much thought about its history or how it advanced from its first commercial use in the 1980’s. While Protein A has maintained its place in manufacturing since being introduced, it has evolved considerably and with recent changes to biopharmaceutical manufacturing it is poised to progress even further.
To learn more about where Protein A has been and more importantly where it is headed, I recently watched a webinar titled “Insights into Recent Developments in Protein A Chromatography,” given by Jonathan Royce, Senior Product Manager, Antibody Affinity Media, GE Healthcare. The webinar provided a good overview on Protein A chromatography and how it is being optimized to fit new manufacturing models and demands. Information was covered about the history of Protein A and its involvement in industrial purification. It went on to discuss ways to optimize the productivity of Protein A and opportunities for further advancement in the area. I have summarized some key points below.
History of Protein A Chromatography
Protein A was first discovered in 1958 by Klaus Jensen when he demonstrated that a cell wall antigen from S. aurens caused precipitation in all 500 normal human serum samples. He thought that this was due to the presence of a carbohydrate substance. In 1962 Löfkvist and Sjoqvist demonstrated that Jensen’s antigen was actually a protein and it was named Protein A in a paper by Grov, et. al in 1964. Then, in 1966 Forsgren and Sjoqvist showed that Protein A binds to the Fc region of IgG molecules.
After decades of additional research it is now know that Protein A is a 42 kDa immunological protein present in the cell wall of S. Aureus bacteria. It is comprised of five homologus Ig-G binding domains, which fold into a triple helix. The stoichiometry of Protein A binding enables one Protein A molecule to bind two IgG molecules. It is currently produced recombinantly in E. coli and the Brevibacillus genus.
How Protein A Works
Protein A binds specifically to the FC region of IgG molecules. Native Protein A also has an affinity for certain variants of the Fab region often called VH3 interaction. Binding occurs at neutral pH, while elution occurs at low pH, except in the case of IgG3. For the latter subclass, both binding and elution occurs at similar pH, and Protein A is less useful in this case.
Evolution of Protein A
Beginning in the 1980’s Protein A chromatography was widely used in protein purification. Protein A has been established as the primary method for purification of monoclonal antibodies. In 2012 over 90% of total mass of mAbs manufactured were purified using Protein A. Protein A is popular because it is fairly simple to use and provides high purity in one step. Because of its universal application it enables a purification platform approach, thus allowing the use of one platform for a suite of different antibodies. The use of a purification platform supports faster process development, standardization of manufacturing operations and ultimately shorter time to market.
However, changes in biomanufacturing needs have necessitated further development of Protein A chromatography to address expanding requirements. As a result, new generations of Protein A have been developed to improve process economy and expand acceptance. As the biopharmaceutical industry has advanced, Protein A has needed to keep up by providing solutions including animal origin free options, higher throughput, longer lifetime to reduce cost, and higher productivity to meet the needs of higher titer culture.
Alkali Stability
One significant improvement that has been made in the new generation Protein A media is the use of alkaline stable ligands, which enable a long lifetime and improved process economy. Today alkaline stable protein A media is the standard in biomanufacturing and the use of base stable ligands has resulted in chromatograpy media with 10X longer lifetimes than previous generations of recombinant Protein A.
Alkali stability provides several benefits beyond capacity including:
- Stable reduction of DNA
- Stable reduction of HCP
- Stable yield
- Stable ligand leakage
The combination of high capacity, good pressure flow capability and alkali stability lead to improvements in cost and process time (Figure 1).
Figure 1
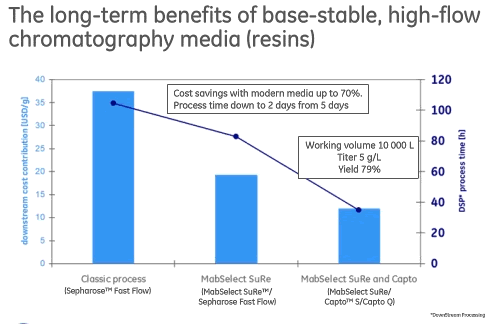
Cost savings that can be achieved with modern next generation Protein A media is up to 70% and process time can be reduced to 2 days from 5 days.
Current Optimization Strategies – Next Generation Protein A Media
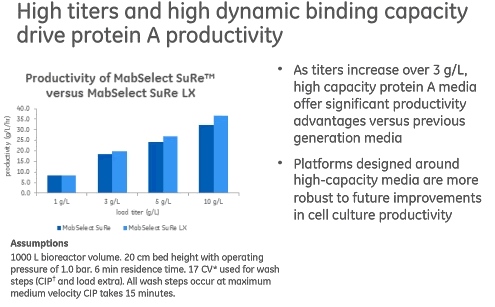
Currently available new generation Protein A media has also addressed several other challenges present in today’s cell culture manufacturing, for instance increasing titers. High capacity Protein A media provides a significant advantage for titers over 3g/L, including increased productivity and improved facility fit (Figure 2).
Figure 2
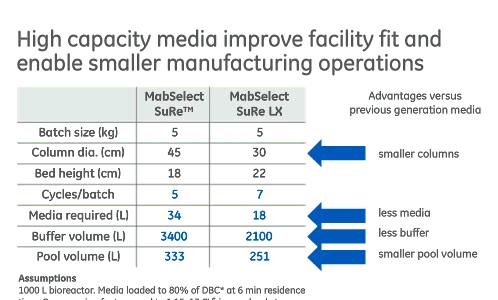
If productivity is acceptable, then the use of a high capacity Protein A media can enable smaller manufacturing operations including smaller columns, less chromatography media, less buffer and reduction in the size of pool volume (Figure 3). These advantages make it an enabling factor in single-use implementation downstream, which provides another set of manufacturing advantages.
Figure 3
Residence Times
Another way to improve purification performance is to optimize the use of Protein A media. One example is to look at varying residence times. Mr. Royce presented GE Healthcare’s work on studying varying residence times to see if the average residence time could be shortened and thus productivity improved. To begin the study, it was understood that most Protein A media are essentially saturated with antibody at residence times longer than 3 minutes, which means that increased residence times don’t result in increased capacity. With GE’s MabSelect Sure LX, they have seen capacity increases at up to 10 minute residence times, so they decided to test this product to see if there could be further gains. Thestrategy employed variable residence times. They divided the loading steps into various parts and worked to find the optimal combination of residence times for productivity.
In the study, 80% of QB10 was loaded for each residence time on MabSelect Sure LX and was compared to a constant residence time of 6 minutes. By varying the residence times there was a significant difference in time needed to complete one cycle, 81 minutes for the variable residence times approach and 120 minutes with a standard residence time of 6 minutes. They were able to save as much as 39 minutes per cycle. This times savings represents a productivity increase of over 40% over fixed residence time and they verified that capacity and purification performance was unchanged. The effect on a typical five cycle Protein A step is that time can be reduced from 10 hours with the fixed 6 minute residence time to 6.7 hours with the variable residence time approach (Figure 4).
Figure 4
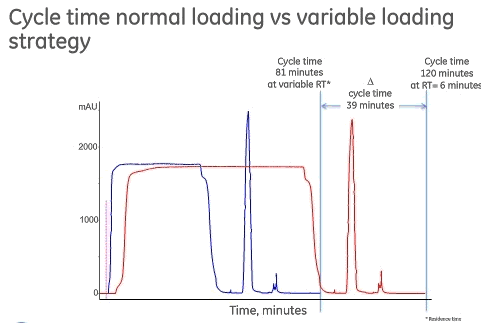
The variable residence times approach can be employed to achieve different goals. Either to reduce processing time while maintaining the same column volume or to reduce column volume but keep the same processing time.
Addressing New Challenges
As biomanufacturing is continually evolving, so must Protein A media. There are some key areas that Mr. Royce identified for improvements including addressing new process methods and new purification challenges.
New Processing Models
One method on the rise is employing either a continuous process and/or a connected process with straight through processing in downstream. The benefits of these processes have also been covered in detail in a previous blog, titled “Continuous Downstream Processing – A Tool to Address Key Manufacturing Challenges.”
New Purification Requirements
New therapeutic entities such as bi-specific antibodies, antibody fragments and antibody drug conjugates are necessitating adjustments in purification methods. In addition, new tools have allowed access to more specific information about these products and as such has resulted in higher selectivity demands including: stricter limits on aggregate and HCP, more focus on product variants, and better analytics which enable more sensitive measurements.
Supply Chain
While media performance and cost continue to be key concerns, raw material supply chain risk continues to be an area of concern due to the single source nature of several chromatography media. While it is a risk, it is also an opportunity for improvement and GE Healthcare has implemented an emergency preparedness plan with a strategic reserve and an identified secondary supplier of up to 90% of material demand. Another key raw material area is the Protein A ligand for which they have two suppliers and agarose beads where a second supplier will come on board in 2016.
Universal Key Improvement Areas for Protein A Media
Mr. Royce discussed 4 key areas where he thought improvements were possible for Protein A Media.
- Higher capacity media – this is the most desired improvement with the largest effect on reducing cost of monoclonal antibody purification, more even than reducing cost of Protein A media itself.
- Faster binding kinetics –for mass transfer Protein A media is grouped broadly into 2 categories built on either large beads or small beads. Large beads offer good pressure flow characteristics, but slower mass transfer. Small beads – faster mass transfer but significant limitations in terms of pressure flow.
- Better base stability – allows for better control of bioburden in manufacturing.
- Better value – Protein A must continue to improve process economy with each successive generation in order to continue to be such a large part of mAb purification.
Mr. Royce went on to explain that improving capacity and mass transfer capabilities could be improved by using ligand design to improve dynamic binding capacity, optimizing bead technology and improving base stability through advanced ligand engineering. Currently GE Healthcare is working on using a high throughput screening method to screen ligand designs for improved characteristics. So far, hundreds of ligand designs have been screened in less than 12 months. In addition to base stability they are also looking at possible benefits to be gained from other ligand modifications and advanced ligand engineering.
In addition to looking at ligand design, they are looking at developing new recombinant variants of Protein A that are more stable than previous generations when cleaned with 0.5 M NaOH. This is important because there are several benefits of cleaning with 0.5 M NaOH for Clean in Place (CIP) operations over using 0.1 M NaOH including: increased viral inactivation, increased bio burden control, and inactivation of endotoxin.
Summary
While Protein A chromatography has remained a constant in monoclonal antibody production since the 1980’s it has had to undergo regular redevelopment and next generation versions to keep up with changing demands in biomanufacturing. These improvements need to continue, if Protein A is expected to continue to maintain a large share of the purification market.
