Viral Safety in Biologics Manufacturing
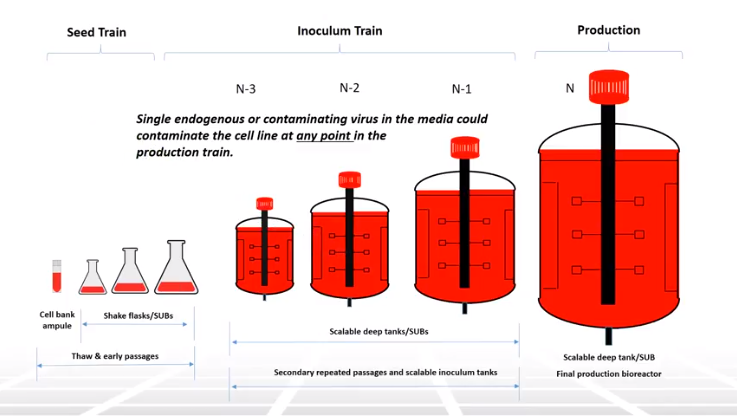
Regulatory bodies have long recognized that viral safety in biologics manufacturing is a top priority. The presence of adventitious agents such as bacteria, mycoplasma, adventitious viruses, endogenous retroviruses, fungi/molds and prions represent a significant threat with patient safety, medicine shortages, costly decontamination as the key concerns. While viral contamination is rare it has happened. To help prevent contamination regulatory guidelines have been established to evaluate viral safety in biologics manufacturing. These guidelines evaluate potential risk and establish testing requirements for the entire biomanufacturing process. This week’s Two Minute Tuesday educational video provides an introduction to viral safety and looks at areas of risk, the regulatory guidelines, testing requirements and critical steps in the biologics manufacturing process. For the full video, please see the video below titled, “Introduction to Viral Safety“.
The Keck Graduate Institute and WuXi Biologics have teamed together to provide technical training videos called Two Minute Tuesday videos for the bioprocessing industry. They will deliver a new video topic every Tuesday that can quickly get you up-to-speed in understanding the complexities of biologics drug development and manufacturing.
For more information on viral safety, please see: