Fine Tuning Viral Clearance Approaches with a Total Viral Challenge Strategy
In this mini-webinar, Michael Burnham, M.S., Senior Principal Scientist, Process Development and Commercialization, WuXi AppTec, presents a viral clearance strategy that focuses on spiking load or starting material based on total viral load instead of percent spike model.
Viral Clearance Strategy
Viral clearance is a critical component of the regulatory submission and approval process. These studies help to define the overall safety of a drug by looking at the capability of a manufacturer’s downstream purification process to eliminate potential viral contaminants.
Mr. Burnham began his talk by explaining how WuXi AppTec has developed a service platform for viral clearance to drive higher log reduction values (LRVs) through the optimization of process steps and study design. By utilizing this platform, WuXi AppTec has successfully completed numerous viral clearance studies with many different products and processes. They have utilized this experience to develop a viral clearance service platform to achieve higher LRVs through the optimization of process steps.
In developing their viral clearance service platform, they employ a number of tools to achieve targeted LRVs, and have incorporated these tools into their study design process. These tools include:
- Large-Volume Testing and Plaque Assays
- In processes where little to no virus is expected, testing extra volume can add more log reduction values (LRVs) to increase total clearance values by increasing the assay sensitivity.
- Ultra-Purified Preparations of Virus Spikes
- Purification techniques result in high quality, high titer virus stocks
- WuXi AppTec has developed unique purification processes for multiple types of viruses that has significantly improved the quality of these virus preparations
- Spiking highly purified virus into initial load samples has shown to improve performance of many process steps
- Target LRVs using Total Viral Load Challenge
- Improved spiking strategy: target a specific LRV per step
- Challenge based on virus titer
Mike went on to explain that despite the success with that they have had in viral clearance, they were looking to devise and implement a new strategy that would provide more consistent performance for process steps within replicate runs as well as across multiple product platforms. He went on to explain that the new strategy focuses on spiking load or starting material based on total viral load instead of a percent spike model.
Viral Removal Filtration
Mike then began discussing virus removal filtration and their associated challenges. He explained that viral removal filtration (VRF) is widely considered a robust process step, able to remove multiple logs of many virus types. However, without careful design of viral clearance validation, there can be challenges with VRF. Several factors including parvovirus breakthrough, virus stock interactions, and flow-through impact or plugging, can have a significant influence on the scale down model, thus potentially impacting the regulatory submission. So the question Mike and his team began asking was – how do we better control viral clearance studies to optimize results?
To begin to answer this question, WuXi AppTec’s technical team evaluated WuXi AppTec’s viral clearance database for examples of unexpected or variable data. They focused on the virus removal filtration step. What they found was that higher spike levels in viral challenges of 9 logs or higher with MVM and PPV resulted in variable runs or lower than expected LRV. Breakthrough of parvoviruses was observed with multiple filter types, however, this was not observed with other ultrapure, larger viruses – retro, herpes, or reo-viruses.
When looking at lower viral challenges of 7.5-8 logs during viral clearance studies, there was more evidence of success viral clearance with complete removal of these small parvoviruses. Viral clearance studies that utilized a challenge of 8 total logs of parvovirus exhibited more consistent LRVs and the majority of the runs resulted in complete removal with little variance between replicates.
They were able to demonstrate that spiking based on total viral load was a more effective method. Historically, viral challenge studies have typically utilized the percent spike model, in which load materials were challenged with a specific percent spike for all steps evaluated in the study. However, not all virus preps have the same titer or level of purity, and spiking based on a percent spike could lead to variable data due to these differences in virus titer and quality. Utilizing a total viral load challenge for viral clearance studies enables consistency between runs and between multiples studies and projects. It is a more scientifically‑sound approach for optimizing processes for robust virus clearance. WuXi AppTec has used this total virus load strategy for the past four years with much success, and all studies using this method have been reviewed and accepted by the global regulatory bodies.
Mike then explained how they calculate a viral challenge.
Best Practices Viral Clearance Studies
Next, he described a collaboration between WuXi AppTec and Asahi Kasei to address the need for best practices for viral clearance studies to limit or reduce impact on potential robust clearance and find the right spike level. They collaborated to design studies that would demonstrate sufficient retrovirus and parvovirus removal using Planova filters, WuXi AppTec’s Ultrapure spikes and assay techniques under adequate virus challenge conditions. They looked to collectively prove that obtaining sufficient LRVs does not require high virus spiking challenges.
Mike then walked through the study design, which is summarized in Figure 1:
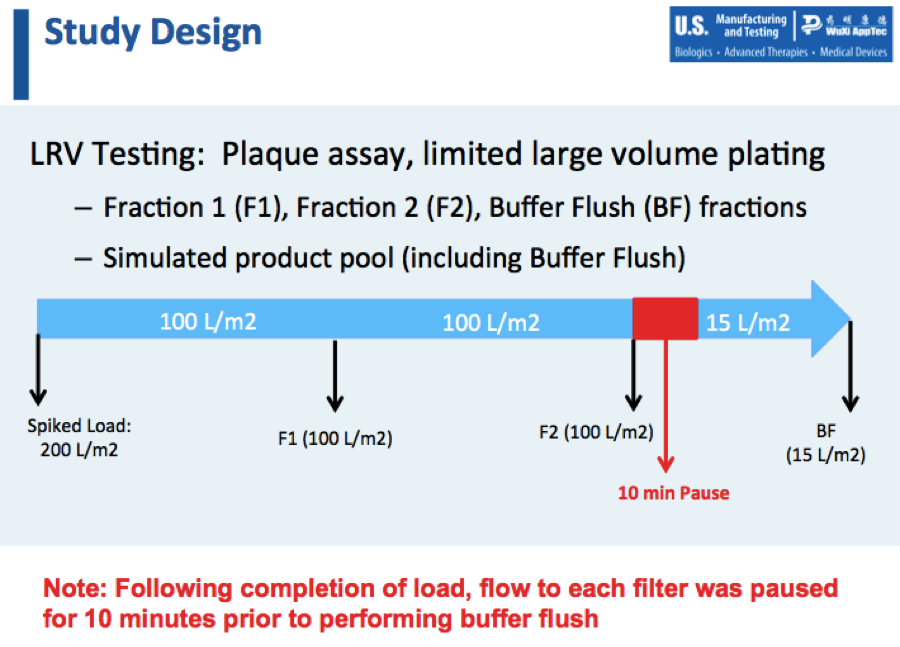
Study Results
During these experiments, Mike shared that both Planova 20N and BioEX filters displayed good retention of virus with no breakthough observed. They obtained high LRVs with minimal viral input. There was little impact on flux compared to an unspiked run. Most importantly there was no LRV impact from low operating pressure or process pause.
In Summary
The data from these studies supports both WuXi AppTec and Asahi’s spiking strategies. Key takeaways include the importance of controlling filtration experiments by limiting spike to ≤ 11 logs / m2 of membrane. Also, best results were obtained when there was a reasonable LRV target and LV testing was utilized to achieve it.
For full data and more information, please watch Mr. Burnham’s presentation below:
About the Presenter:

Michael Burnham, M.S., Senior Principal Scientist, Process Development and Commercialization, WuXi AppTec
Michael Burnham is a Senior Principal Scientist with WuXi AppTec’s Process Development and Commercialization Department. Mike has over 20 years’ of biosafety and bioprocessing expertise, serving as a technical and regulatory advisor for the design and execution of viral clearance studies. He has also developed novel purification strategies for multiple virus types to improve the growth, assay and quality of viruses used in Viral Clearance studies, as well as developing and validating assays for new emerging viruses.
Currently, Mike and the Process Development team are developing processes for the production, purification and quantification of recombinant Lentiviral and other gene therapy vectors in preparation for commercial manufacturing.
Mike earned his Bachelor’s in Biological Sciences at the University of Delaware and his Master’s in Biological Sciences at Rutgers University.
