
Fortem: A platform film built for bioprocess
The increase in adoption of single-use bags for manufacturing biopharmaceuticals has been driven by the many advantages it offers. However, the materials of construction can be more complex than conventional stainless steel vessels. Single-use containers are constructed from plastic films, which are often composed of several layers of polymers with additives for processing and performance.
The diversity of applications for single-use containers requires film that can achieve a wide variety of performance attributes such as mechanical strength, flexibility, biocompatibility, and suitable gas barrier properties to name but a few. The right balance of chemical composition and film architecture is critical for achieving desired performance across many applications.
With single-use bags at nearly every stage of bioprocessing, it is important that if different films are used for different single-use products, individual product qualifications must be conducted. Often this means several different single-use bag qualifications to cover the entire process.
To improve film quality and simplify the qualification process, GE Healthcare is launching their Fortem™ one film, a single-use platform film at Interphex this week. This platform film will be used for their entire portfolio of single-use products. Thus enabling a single qualification for multiple single-use bags in a process.
Fortem was designed specifically for bioprocess applications and GE Healthcare partnered with Sealed Air Corporation, a manufacturer of primary films for pharmaceuticals, in the design and manufacturing. In addition, GE material science experts worked closely with customers to design a film that would meet the critical quality attributes required for a platform bioprocess film.
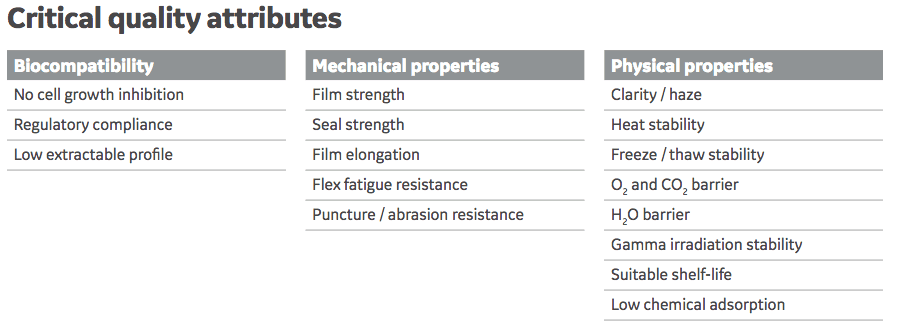
Other Key attributes of Fortem include:
- Well characterized – specific analytical work to identify and control degradative products.
- Developed in accordance with the latest industry guidance – in particular the BPOG extractables testing protocol.
- Qualified using new methodologies for film failure – included tests for flexural fatigue, weldability, and abrasion resistance.
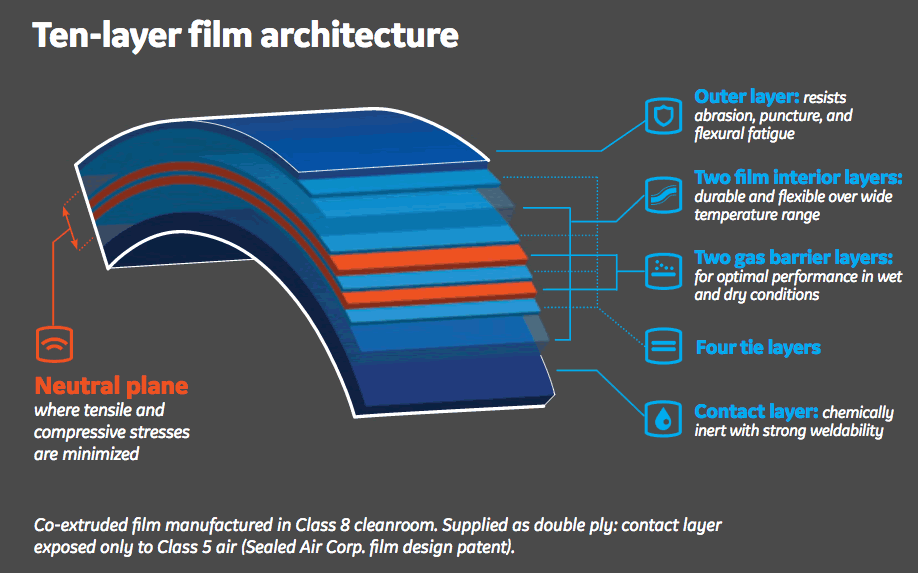
Fortem Architecture
The film’s ten layer architecture includes a contact layer that is composed of an olefin resin blend with a low extractables profile and strong weldability characteristics.
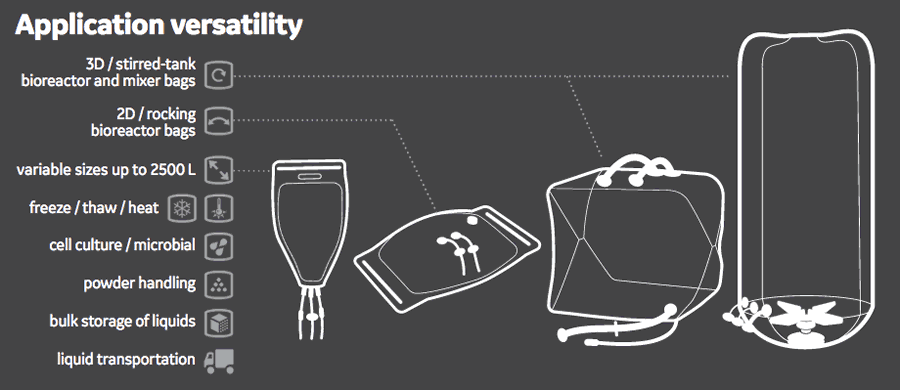
Suitable for the Entire Process Train
Security of Supply
To ensure consistent performance and protect from supply disruptions, GE Healthcare has developed a business continuity plan which is ISO22301 accredited. It includes robust site preventative and recovery plans, a ten-year strategic supply agreement, and strategic safety stocks in both raw materials and finished film.
For more information, please see
Or visit Fortem
