Full Adeno Associated Virus (AAV) Capsid Enrichment Using Mustang® Q Membrane
Mustang Q XT step gradient elution demonstrated full capsid enrichment of 4-5-fold and produced elution fractions with ~100% full capsid content
Adeno-associated virus (AAV) vectors are becoming increasingly important gene delivery platforms for the development of gene therapies for several reasons. They are relatively safe with low immunogenicity, and demonstrate high gene transfer efficiency and stable long-term expression in both dividing and quiescent cells. As well, a number of AAV serotypes exist that differ in their tissue tropism, or the types of cells they infect, making AAV a very useful system for transducing specific cell types. Typically, AAV production occurs within a packaging cell line, such as HEK293T cells, where plasmid containing the gene of interest (GOI) are assembled into a protein capsid shell. A current challenge in manufacturing is that the encapsidation efficiency for AAV is highly variable, which produces a heterogeneous mixture of AAV particles that contain the GOI (full) and particles without any genome (empty) or with fragmented, non-functional DNA (partial). Because only the full AAV capsids can exert a therapeutic effect in vivo, the other capsids are viewed as a process impurity and removing them is a necessary step in downstream purification process to achieve a consistent drug product. Existing methods to purify full AAV capsids like analytical ultracentrifugation (AUC) are effective yet have limitations in throughput and cost for a manufacturing environment, which has driven the development of more scalable methodologies for AAV purification.
A recently published application note from Pall, describes a novel method to enrich for full AAV capsids during production processes using the Mustang Q membrane anion exchange chromatography. These ready-to-use chromatography membrane adsorbers have a wide range of bed volumes (1 mL to 5 L) and are designed for capturing targeted molecules or to remove impurities across all bioprocess scales. The application note outlines the general methods and buffer compositions utilized in developing the new elution strategy. Full AAV capsid purification or enrichment was tested with three AAV serotype 5, 8 and 9 using the 0.86 mL Mustang Q XT membrane in the Acrodisc® format, and the scalability of the new purification method was evaluated with AAV5 using the 5 mL Mustang Q XT capsule on the Cytiva ÄKTA™ avant chromatography system (Figure 1). Instead of traditional linear gradients, a novel step gradient elution with small (~1 mS/ cm1) conductivity steps was used. This separation strategy produces a series of discrete elution peaks, which can greatly simplify AAV capsid purification processes and thereby accelerate process development.
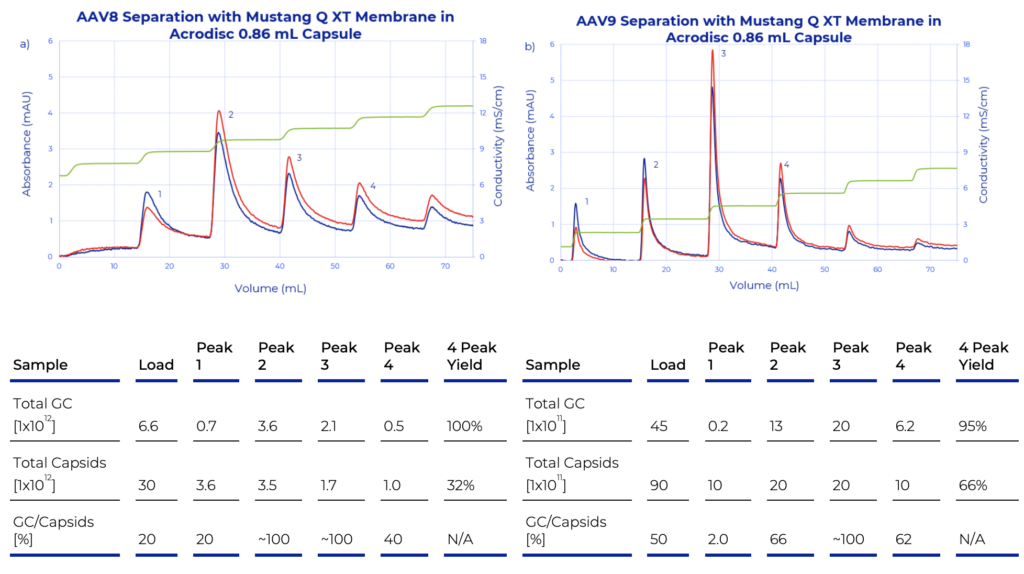
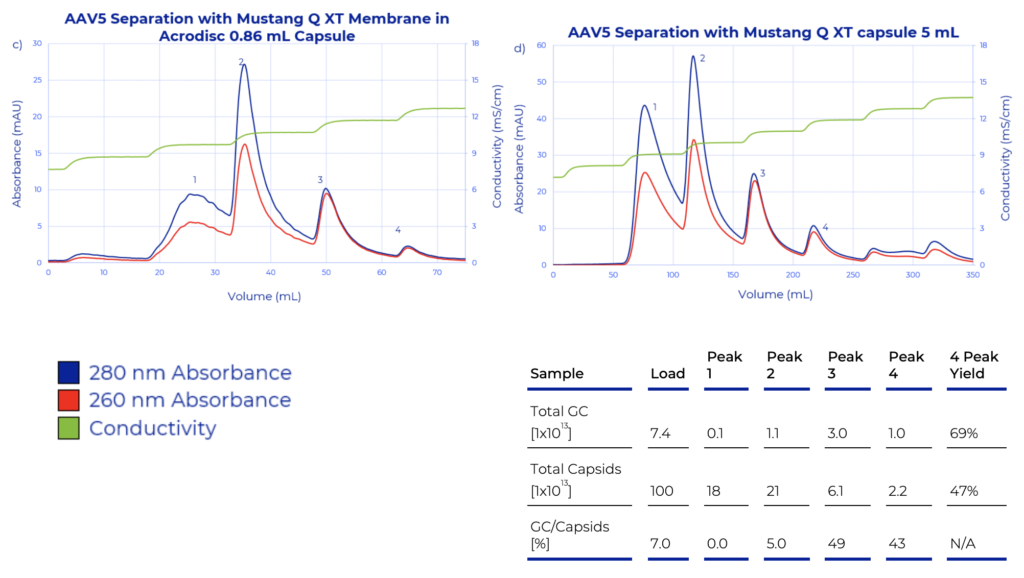
AAV capsid content during purification was assessed in real-time using UV absorbance (A260 nm/280 nm) and confirmed with capsid ELISA, and digital drop PCR (ddPCR). The ratio of these two measurements (ddPCR/ELISA) x100) provides a rough estimation of the percentage of full capsids within an elution fraction. Using the Mustang Q membrane with the step gradient elution demonstrated full capsid enrichment of 4-5-fold and produced elution fractions with ~100% full capsid content. The data strongly suggests this strategy can be used as a platform process step across AAV serotypes. Together with the demonstrated scalability of the method to 5 mL Mustang Q capsules (shown with AAV5), this approach offers a suitable alternative to other purification methods for use in a biomanufacturing setting.
Moreover, the Mustang Q membrane can be operated at high flow rates of up to 10 membrane volumes per minute (MV/min), highly desirable for loading onto anion exchange because typically, large dilutions after the affinity step are needed to raise pH and lower conductivity prior to anion exchange. The low backpressure at high flow rates enables large dilution samples to be loaded quickly onto the column. This rapid chromatography performance along with the elution methodology is well suited for AAV process intensification. By adjusting the conductivity step gradient, optimization of the protocol is possible for different serotypes making it a truly scalable platform technology. This method of applying a conductivity step gradient provides superior enrichment of full AAV particles compared to a conventional conductivity linear gradient elution and using a membrane device is ideal for process development and manufacturing environments.
To read the application note in full, please visit: Full Adeno Associated Virus (AAV) Capsid Enrichment Using Mustang® Q Membrane
