
Increasing Downstream Bioprocess Efficiency and Overcoming Bottlenecks
In a recent white paper, the issue of improving downstream efficiency was explored. The paper, “Unlocking the Potential for Efficiency in Downstream Bioprocess,” published by GE Healthcare Life Sciences, described techniques like continuous processing, in-line conditioning buffer preparation, and system automation as tools to improve the overall efficiency of downstream processes while at the same time eliminating bottlenecks and facility fit issues. I have summarized the highlights of the paper in this article.
Biomanufacturing is constantly evolving due to changing industry demands and new technologies that enable advancement. Industry goals are now primarily focused on reducing cost and improving throughput, productivity, time to market and flexibility. These goals must be met whilst maintaining the highest levels of product quality and safety requirements. With increased titer, downstream processes have had to manage higher titers and greater impurities than they were originally designed for. Thus downstream processes must also be improved to create an entire manufacturing process that is more streamlined and meets industry goals.
Downstream Challenges
The white paper authors identify several challenges facing downstream as a result of new demands and increased upstream titers.
Challenges include:
- The need to be able to scale up or down based on demand.
- Improving flexibility and increasing facility fit by reducing equipment size and the overall footprint.
- Adjusting to the change in product coming from upstream, particularly the increase in titers and impurities in a cost-effective way.
- Reducing downtime and hold steps.
- Increasing facility use, productivity and throughput.
Increasing Downstream Efficiency and Addressing Challenges
The authors go on to describe how the use of process intensification can overcome these challenges. In the paper they present a toolbox approach where process intensification tools can be applied independently or as a group to improve problem steps. By looking at process intensification as a toolbox, you permit the most flexibility in terms of addressing challenges, thus adding improvements only in areas that are needed and in a targeted way to address specific problems.
Examples of process intensification tools include:
- Improve column handling
- Eliminate cleaning and validation
- Simplify buffer preparation
- Increase system integration and automation
- Increase productivity with connected continuous process
Process Intensification Tools
Improve Column Handling
Column packing utilizes time and resources and can result in a suboptimal packed bed. The successful packing of a column depends in large part on the operator’s experience. The authors describe this process as an art rather than science. In order to achieve better results and incorporate more science than art, the authors suggest automated column packing. Automated columns can reduce time, labor cost and inconsistency (Figure 1).
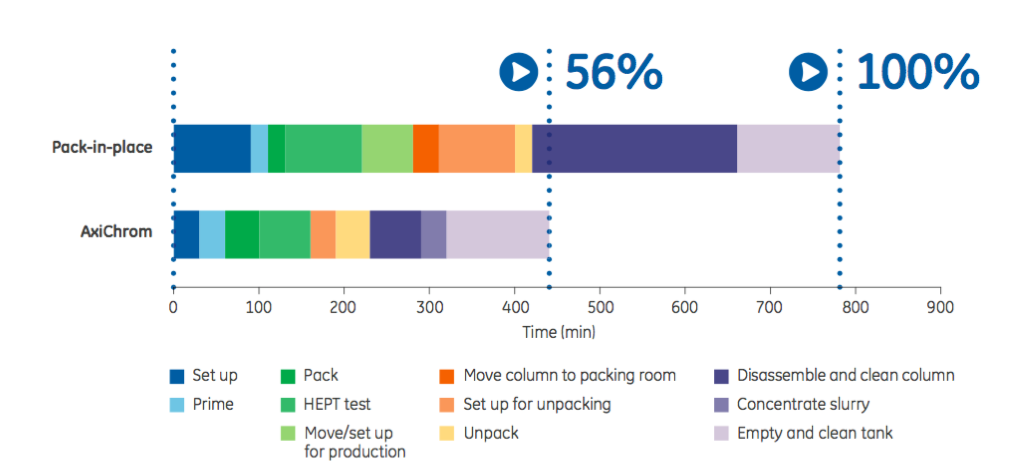
The authors provide the example that GE Healthcare’s AxiChrom™ columns allow one operator to successfully pack a column quickly regardless of their previous experience due to the preprogrammed, verified, and automated packing methods together with a purposeful column design.
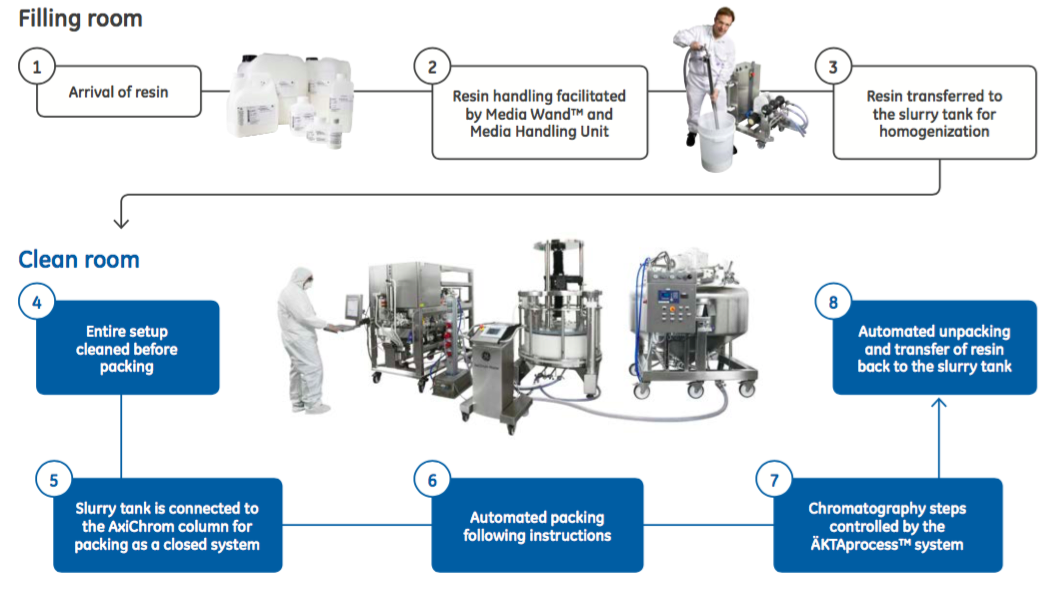
In addition, bioburden control can also be improved with automated packing. Column handling and design can increase the risk of bioburden contamination. The authors describe how AxiChrom has several features designed to maintain bioburden control. These include the planar distribution system and the priming groove. “By connecting the column, slurry tank, and chromatography system in a closed system, manual handling is kept to a minimum and the contamination risk is further decreased (Figure 2). This setup also minimizes resin loss, enabling more efficient use of the resin.”
Eliminate Cleaning and Validation
Cleaning and removal of impurities is an important part of obtaining the full value of resin. However, cleaning and validation are also very time consuming and must take place before, during and after a campaign. One of the bottlenecks in downstream is waiting for results from cleaning verification, but implementing single-use technologies can eliminate this bottleneck.
Single-Use in Downstream
There are several opportunities to implement single-use in downstream. Chromatography systems with single-use flow paths, plug-and-play chromatography columns and membranes, as well as pre-sterilized filters and tubing all offer solutions that don’t require cleaning and validation.
Other benefits beyond eliminating cleaning and validation include increased flexibility, faster turnaround time between batches and ultimately quicker product release.
Single-use Tools
The authors provide several examples of ready to use single-use tools for downstream processing.
- Single-use flow path chromatography
GE Healthcare’s ÄKTA™ ready is an example of a single-use flow path chromatography system designed for process scale-up and manufacturing. The system has ready-to-use, disposable flow paths that eliminate the need for cleaning and validation between products and batches.
- Pre-packed chromatography columns and single-use membranes
Another area where single-use can be implemented is in columns and membranes. For example, GE Healthcare has their ReadyToProcess™ columns that are prepacked, prevalidated, and prequalified. Chromatography preparation times can be significantly reduced by using pre-packed columns.
- Single-use adsorber membranes
Another opportunity for single-use implementation can be found in adsorber membranes. These can be a viable alternative to packed bed chromatography depending on the scale and batch frequency. For example, the authors explain how the ReadyToProcess Adsorber single-use membranes “can be easily switched in and out of manufacturing processes and are of high value in low- to mid-frequency manufacturing setups and in multi-product facilities. The choice between packed bed and membrane chromatography, however, is always case-dependent and requires careful analysis.”
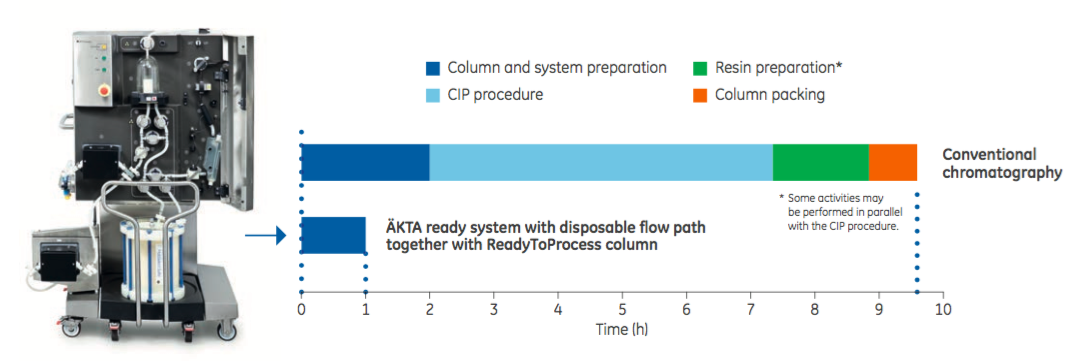
One example of the benefit of combining process intensification steps is provided in Figure 3. By coupling the ÄKTA ready system single-use flow path chromatography with ReadyToProcess columns, downtime between products and batches is significantly reduced. Data shows a nearly ten-fold reduction in set up time when compared with conventional set up. This system could address critical bottlenecks particularly in multi-product facilities.
Simplify buffer Preparation
In-line conditioning vs. in-line dilution
Buffer preparation in downstream is significant. This presents several challenges including: time and resource consumption, capital investment and maintenance of tanks and the large footprint required. One solution is in-line conditioning, which simplifies buffer preparation by reducing the number of tanks, buffer solutions, time/resources and footprint.
The authors provide an excellent explanation of the differences between in-line dilution and in-line conditioning. “In-line dilution means diluting concentrated buffers with water. pH needs to be preadjusted, or adjusted after dilution. In-line conditioning means buffers are formulated using concentrated stock solutions of the required acid, base, WFI, and salt. A wide range of buffer concentration and pH solutions can be created. Conductivity and pH adjustments are automatically done throughout the process.”
In-line conditioning can also decrease variability in buffer preparation because common ion effects are avoided in in-line conditioning as single-component stock solutions are used. Thereby enabling higher concentrations and smaller volumes. The inline conditioning systems from GE utilize a dynamic flow of pH, flow and conductivity. These customized, large-scale manufacturing systems can either be used as a buffer preparation station, or be integrated as part of a chromatography or filtration system.
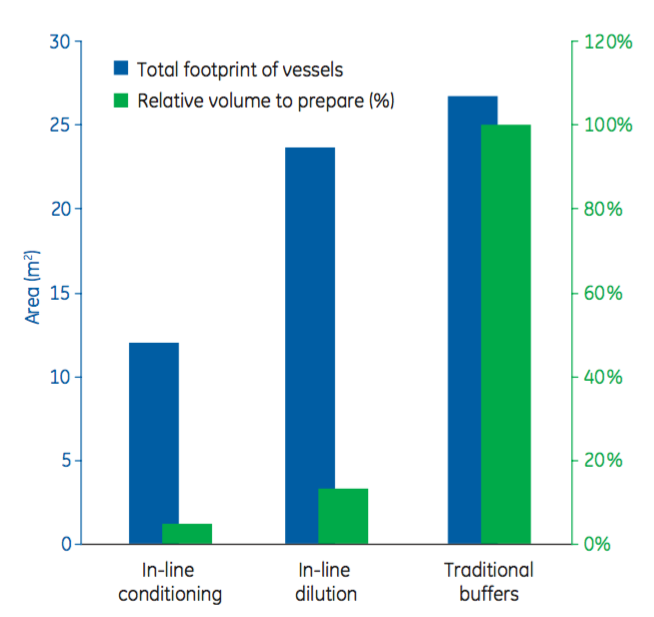
The authors then explain the process economy gains that can come from in-line conditioning. In Figure 4, the authors show results from process economy calculations done on four chromatography steps in an albumin purification process. “The results show that the footprint was reduced by about 50% for in-line conditioning, in combination with single-use stock solution bags, compared with prepared buffers and in-line dilution. Furthermore, in-line conditioning decreased the volume of solutions to prepare by about 95% and 60% compared with prepared buffers and in-line dilution, respectively.”
Increase system integration and automation
System integration and automation is an important part of the acquisition, management and use of data. Data can drive process improvements, but in order for it to be effective, there must be a platform in place to collect and manage the data. In order for this process to work efficiently equipment must integrated with each other and there must be an automation platform in place that can collect and analyze data from multiple sources. Achieving this level of automation frees operators to spend time on process analysis, exception handling, and compliance support.
In the white paper GE describes how they offer a variety of products suited to today’s manufacturing environments. The FlexFactory platform is for example available based on either Wonderware™, together with Allen-Bradley™, or DeltaV™ automation platforms. Each platform is capable of integrating bioreactors, purification systems, and mixers.
Increase productivity with connected or continuous processing
Continuous processing is a very successful model in many other industries. As a result, the biotech industry has begun investigating this model in more depth and has started to incorporate certain aspects of continuous processing into biopharmaceutical manufacturing. One area where it can be particularly helpful is in addressing rising titers and improving facility fit challenges.
Continuous processing has been shown to effectively increase productivity and facility utilization in several instances. Some reasons to consider a continuous process or semi-continuous option include addressing facility fit issues and improving facility flexibility and utilization. Product quality concerns, particularly for unstable products where delays in processing could negatively affect quality, can also be managed more effectively with continuous processing.
While implementing an end-to-end continuous process in biopharmaceutical manufacturing may still be many years away for most companies, there are some real advantages in utilizing portions of a continuous process in certain areas of manufacturing. Continuous processing technologies can provide solutions to specific challenges and can drive the implementation of a continuous or connected process.
Innovative continuous downstream technologies including straight-through processing (STP) and periodic counter-current chromatography (PCC) can be used to solve specific challenges that exist in traditional batch processing.
Straight-through processing (STP)
With straight-through processing chromatography steps are connected in series with in-line adjustment of process conditions between columns to ensure optimized loading conditions (Figure 5).
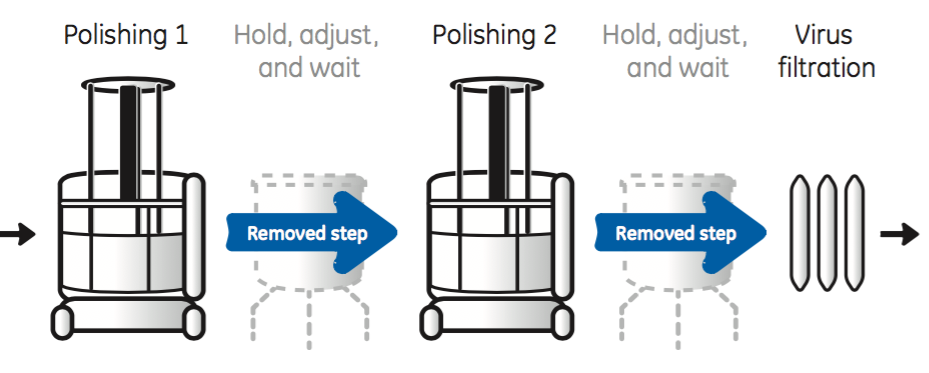
As shown in Figure 5, straight through processing eliminates the need for intermediate conditioning steps required in traditional batch processes. Removing these steps results in minimal need of hold-up tanks and an increase in process throughput.
Periodic Counter-current Chromatography
A clear benefit of using PCC Chromatography is that you can utilize the full potential of the chromatography resin by employing a three or more column operation. In PCC, you begin with Column A and you load it to 60-80% breakthrough, then you disconnect that column and follow with wash, elution and equilibration. While that is happening you switch to Column B and then load that to breakthrough and follow the same steps switching to Column C, etc. By the time you get to Column C, Column A is ready to come back online and you can repeat the process, thus creating a continuous purification process. PCC increases the use of available resin and enables smaller equipment footprint and shorter processing times compared with batch processing.
One key question is – What happens if the breakthrough curve changes? For this you need dynamic control, which automatically accounts for variation in feed concentration and/or chromatography resin lifetime effects. It also enables operation at optimum column loads. Overall it can improve chromatography resin lifetime by limiting exposure of the resin to feed depleted from the product.
For example, the ÄKTA PCC pcc 75 system from GE supports continuous chromatography in a three- or four-column (3C or 4C) PCC setup. In this setup, column loading can be controlled either statically, based on time, or dynamically, based on UV absorbance. By using dynamic control the system operates under process conditions where either feed concentration or chromatography resin capacity varies. As the authors point out, there are several advantages with this, for example, eluate consistency can be ensured when the capture step is connected to a perfusion culture with varying titers.
Implementation of Multiple Process Intensification Tools
To demonstrate these tools in action, the authors provide an example of how a downstream process could be intensified to increase efficiency based on a facility setup at a GE customer site.
“With the advances in upstream bioprocessing, the same amount of protein can be produced in a 1000 or 2000 L bioreactor, at higher titer, as in a 10,000 L bioreactor. Up to six of these bioreactors can feed one intensified downstream process enabling a yield of a ton of mAb each year, at a titer of around 6 grams per liter.
In order to achieve these results, the intensified downstream process would include modern high capacity chromatography resins. It would also require an automated column for fast preparation and maintenance time. In-line buffer preparation and smart automation, allowing connecting steps without hold time, would significantly reduce the tank footprint. The described set-up also provides a more flexible solution as the number of bioreactors can be adjusted to a gradually increasing demand or new products.”
Summary
In summary, there are many new, enabling technologies to increase efficiency and decrease bottlenecks in downstream. The approach of viewing each of these as a tool to be used to solve specific challenges either in tandem or on their own seems to be a logical approach. In this manner, the entire process does not need to be overhauled all at once, but rather in steps as needs arise. It is also important to keep these tools in mind while designing new facilities as the flexibility that these technologies provide will help create a facility that can change with time to meet ever evolving industry needs.
To learn more and to read this paper in full, please see “Unlocking the Potential for Efficiency in Downstream Bioprocess”.
Related Reading:
- Gain Productivity in Protein Purification through Column Loading Optimization
- Protein A – Evolution and Optimization Strategies
- Continuous Downstream Processing – A Tool to Address Key Manufacturing Challenges
