‘Jetting’ Technology for Manufacturing Agarose Beads with Enhanced Performance Characteristics
The vast majority of chromatography resins designed for large-scale bioprocess chromatography separation are produced using traditional batch emulsification in conventional stirred-tank reactors. In these cases, the size of the beads formed in the reactor is a function of the shear force generated by the impeller. The faster the impeller speed, the smaller the beads are. As a result, there is a wide particle size distribution of the manufactured beads. Furthermore screening is required to remove coarse and fine beads, which detract from column performance. This screening is extremely time consuming particularly for smaller beads (less than 65 µm). The smaller the bead being produced the lower the achieved yield so realistically one cannot make beads financially viable less than 40 µm. It also adds high costs due to the additional time in the manufacturing facility with large volumes of waste from the fine and coarse beads. Even after this screening, the resin will still have a relatively wide size particle distribution.
‘Jetting’ technology
Over the past decade Purolite has developed and introduced proprietary ‘jetting’ technology for continuous manufacturing of uniform particle size synthetic polymer beads. This innovative manufacturing process removes the need for extensive and costly screening and improves yields significantly. The use of ‘jetting’ technology has been so successful, that today Purolite produces 1,000 m3/year of synthetic resins using this technology. These resins are used in a range of different applications including industrial and analytical chromatography, pharmaceutical and food catalysts, and for generation of high-purity water.
Agarose bead manufacturing using ‘jetting’ technology
To address the challenges with agarose bead manufacturing, Purolite has applied their proprietary ‘jetting’ technology for manufacturing agarose beads in a dedicated, state of the art facility. Based on data provided by Purolite, resins manufactured using this technology demonstrate enhanced performance characteristics including: better pressure/flow properties, improved resolution, and uniform particle sizes. Purolite uses its ‘jetting’ technology to provide a more efficient and quicker method for manufacturing agarose beads with considerably less waste generated.
After reading about the ‘jetting’ technology I was able to interview Purolite Life Sciences’ Agarose Sales Director, Chris Major, to learn more. Please see the transcript of our interview below.
What led you to apply your ‘jetting’ technology to the manufacture of agarose beads?
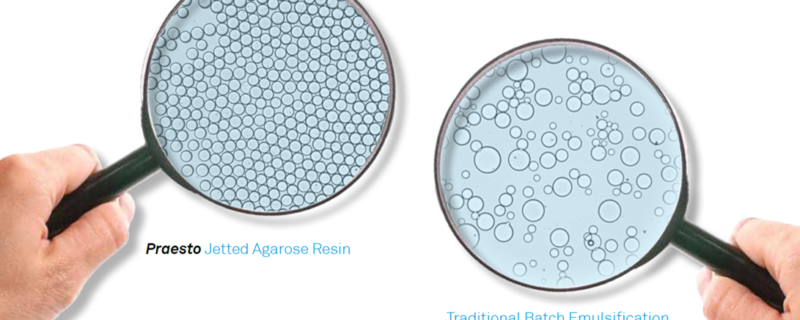
For over 35 years Purolite has led the way in resin innovation. ‘Jetted’ technology was developed to address customer requests for uniform resin particles. For instance, the particle size range of a 35 µm jetted bead is 95% within 25-50 µm with uniform co-efficiencies of < 1.3 compared to a conventional emulsified bead of 45 µm which has a much wider range, with 95% within 25-100 µm with a uniform co-efficiencies of 1.9, the pictures speak for themselves.
What can customers expect from the new ‘jetting’ technology manufactured agarose beads?
First ‘jetted’ agarose beads will allow our customers to deliver even more efficient processes and increase productivity thereby. Second, due to the environmental benefits of ‘jetted’ agarose enables our customers achieve their corporate goals of sustainability. Third, this allows Purolite to bring to market beads smaller than 45 µm that would otherwise be uneconomical to manufacture at the bioprocess scale. Forth, ‘jetting’ technology will dramatically reduce lead times when compared to conventional agarose manufactures which are often upwards of 9 months for large volume commercial batches.
It is clear from my reading and my discussion with you that Purolite is committed to the environment. Can you please tell us more about how Purolite works to provide environmentally friendly solutions?
Purolite is fully committed in leading the way with environmentally friendly processing. The use of ‘jetting’ technology eliminates this need for screening and is also is more environmentally friendly as it removes the need for organic solvents during the emulsification process. During the early part of 2017 Purolite was awarded ISO certification 14001:2015 for environmental management.
Can you tell us more about your new-dedicated facility?
Our new ‘jetting’ manufacturing facility, which measures at over 2000 square feet, is in addition to our 100,000L agarose manufacturing facility which we announced in early 2016. Both will be located at our UK Welsh facilities Centre of Excellence under our ISO 9001:2008 accreditation.
What impact does the reduction of waste product have overall?
First of all, ‘jetting’ produces less waste and has lower environmental impact. Secondly, it will help Purolite manage their cost base with the ability to pass back savings to our customers to allow them to develop more affordable and accessible medicines.
