
New KANEKA KanCapA™ 3G for Improved Binding and Milder Elution of Therapeutic Antibodies
Protein A is by far the most common purification method in biopharmaceutical manufacturing. Due to its high affinity and selectivity for therapeutic antibodies, high purity can often be reached in a single step. With the expanding market for therapeutic antibodies, pressure to reduce the cost of pharmaceuticals, and increases in upstream production titers; Protein A improvements have been required to meet industry demands for improved downstream purification efficiency.
To address both increased efficiency and lower production cost, Kaneka has launched its new Protein A resin, KANEKA KanCapA™ 3G. Utilizing novel protein design technology, Kaneka has developed an improved ligand that has resulted in a 50% increase in static binding capacity compared to their standard product. In addition to improved binding capacity, KANEKA KanCapA™ 3G, a cellulose-based Protein A resin, is designed for milder elution conditions and offers unique impurity removal properties.
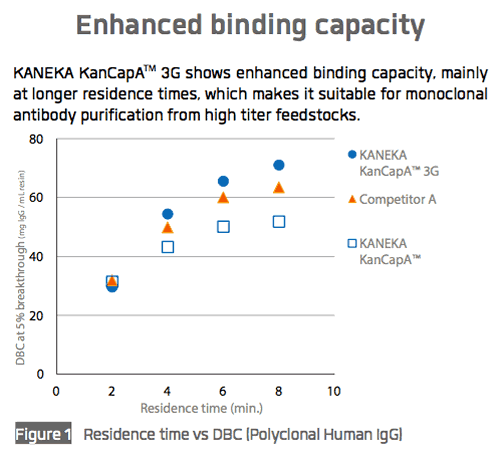
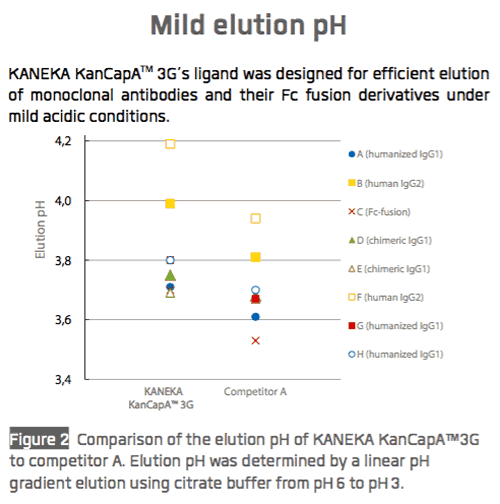
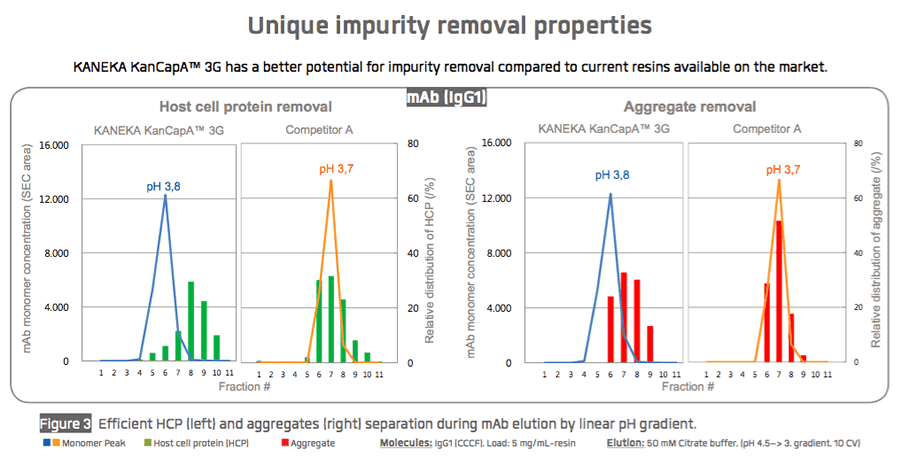
Kaneka recently shared performance data for KANEKA KanCapA™ 3G with respect to binding capacity (Figure 1), elution performance (Figure 2), and impurity removal capability (Figure 3).
For more information, please see – KANEKA KanCapA™ 3G
