Implementing Disposable Chromatography: Technology Fit in Downstream Purification
Fletcher Malcom, Dana C. Pentia, William J. Wilde, James R. Peyser
Summary
Disposable and single-use technologies have become standard in many of the world’s leading biopharmaceutical companies. Faster product changeover, favorable economics, and improved safety have driven this paradigm shift. As with any paradigm shift, overcoming barriers to implementation is critical to the success of pre-packed disposable columns in GMP manufacturing.
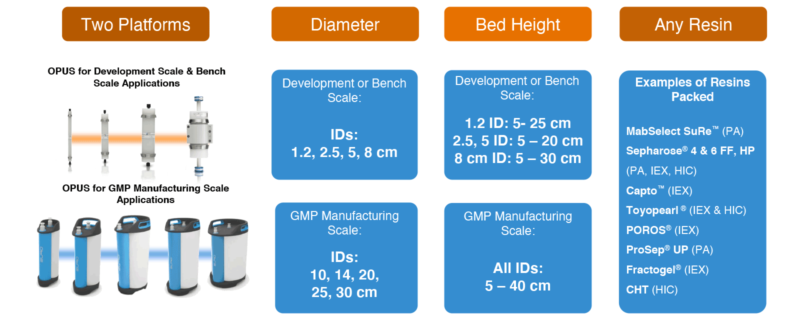
In 2012 Repligen conducted proprietary market research1 to validate the most important barriers and found column size, chromatographic performance, economics, and documentation were the most commonly referenced barriers to implementing pre-packed disposable columns. OPUS® (Open Platform User Specified) columns by Repligen have been intelligently designed and developed for GMP Manufacturing to offer the following:
- Column Size: 9 industry standard diameters available in bed heights ranging from 5 – 40 cm
- Chromatographic Performance: OPUS columns maintain critical purification parameters throughout extensive cycling experiments simulating >100 process cycles
- Economics: An economic model developed with BioProcess Technology Consultants (BPTC) shows OPUS columns save on average $20,000 USD for a small scale clinical campaign
- Documentation: OPUS columns are manufactured under a certified ISO 9001:2008 Quality Management System and come with a regulatory support file and fully qualified certificate of analysis
