
The Future of Biopharmaceutical Manufacturing – Solutions to Address Industry Challenges
Introduction
In September, I was able to attend Cell World 2015 sponsored by Finesse. The conference was focused on the future of biomanufacturing and hosted many interesting talks on the state of the industry, manufacturing strategy, facility planning and utilization and useful new technologies and tools to address the evolving needs of the biopharmaceutical manufacturing industry. I have covered some of the highlights in this blog.
Biopharmaceutical Development and Manufacturing Challenges
There are many challenges facing biopharmaceutical companies as they move a drug from discovery to commercial manufacturing. These challenges drive continual improvements in both manufacturing strategy and the associated tools or technologies used. Some challenges currently facing the industry include:
Pricing Pressures
There is more pressure than ever to bring drug costs down. The desire to reduce drug costs has generated several proposed solutions including government set price controls and the introduction of biosimilars. Pricing issues have also made cost effective, highly efficient manufacturing a top priority.
Speed to Market
With high development costs and competition for the same drug targets, reducing time to market and being first to market with a new product is critical. In many cases companies are already working on next generation products as the initial products are being approved. Expedited regulatory programs from FDA like Fast Track and Breakthrough Designation can give companies an accelerated pathway, provided companies can demonstrate that their treatment provides significant improvement over existing treatment and/or fulfills an unmet need.
Manufacturing for Emerging Markets
Emerging markets have been identified as an opportunity for significant future growth in the pharmaceutical industry. This potential growth is fueled by the manufacturing of products to treat the local population and also in manufacturing products for other developing markets. As such, many companies are increasing their manufacturing efforts in these areas. This includes the growing effort for manufacturing in BRIC (Brazil, Russia, India and China) countries. However there are difficulties with beginning operations in these countries that primarily involve the experience and training of regulatory agencies, engineering and construction firms, management level employees and operators. Thus single-use facilities with a high level of automation are preferred, as they simplify the process and provide facility build out that is faster and requires lower capital investment.
Novel Biopharmaceuticals Require Different Manufacturing Competencies
Novel treatments including personalized medicine with companion diagnostics, antibody drug conjugates, stem cell therapies, and CAR T-cell therapies are some examples of therapeutics moving through clinical pipeline that require a more complex manufacturing strategy compared to standard monoclonal antibody treatments. These products require more extensive planning and coordination to execute. In many cases new equipment and technologies must be created or adapted to meet special manufacturing requirements.
One example of the development of new equipment was presented by Ohad Karnieli, Ph.D., VP, Technology and Manufacturing, Pluristem Therapeutics. He described how Pluristem developed a thawing device to safely thaw their stem cell therapy at the clinical site. They realized that with this type of therapy they had to look at the whole chain and that their job didn’t end when the product left the facility, it wasn’t complete until it was delivered correctly to the patient.
Biosimilars
Biosimilar manufacturing presents a host of new challenges to consider. A biosimilar company’s primary manufacturing goal is to show comparability to the reference product, however they have no detailed description of the development process of the reference product so they must approximate process conditions. While they typically have better manufacturing technology available compared to the innovator company at the time, this is often counterproductive to achieving comparability. Improvements in manufacturing efficiency may change the process enough to create variations in the final product. There is a significant amount of testing that needs to be done in order to show comparability. Above all, costs need to be kept to a minimum with many estimating that on average a biosimilar should provide a reduction in cost of 20-30%. Speed to market is also critical here as many companies are focused on the same target products.
Increased Need for Flexibility
In recent years, there has been an increasing need to produce more products at smaller volumes. This has occurred for several reasons. Product yield increases and diversification of product portfolios has reduced the need for 10,000 – 25,000 liter batches and allowed for the opportunity to manufacture multiple products in the same facility. Traditionally turnaround time, cleaning, sterilization, and validation would have made it challenging to produce many products at the same facility. However with single use systems, cleaning, sterilization and validation are greatly reduced or eliminated. This also enables the move toward smaller market or orphan drug products that have reduced product demand.
Innovative Tools and Strategies to Meet Manufacturing Challenges
Perfusion and Continuous Processing
Continuous processing has proved a very successful model in many other industries. As such, there has been a growing interest in utilizing continuous process concepts in the manufacture of biopharmaceuticals. Furthermore, certain continuous technologies have already been incorporated into existing biopharmaceutical manufacturing with many benefits. For example, in several applications, perfusion culture has provided benefits over traditional fed-batch processing and has been used successfully for many years to manufacture unstable proteins. These benefits can include higher yield, increased speed, cost savings, effcient facility utilization, better scalability and improved product quality and stability.
While implementing an end-to-end continuous process in biopharmaceutical manufacturing may still be many years away for most companies, there are some real advantages in utilizing portions of a continuous process in certain areas of manufacturing. Continuous processing technologies can provide solutions to specific challenges and can drive the implementation of a continuous or semi-continuous process.
One example of the incorporation of perfusion culture into manufacturing was provided by Jon Coffman, Ph.D., Director of Engineering with Boehringer Ingelheim. In his talk he discussed the challenge of being first to market and a possible solution. He presented a strategy of using perfusion culture to develop fast, small, and scalable manufacturing for regulatory and toxicology studies. This would provide a real advantage in terms of speed if a candidate were successful in initial studies because there wouldn’t be a wait time for additional material to be manufactured.
His group discovered that when they implemented a perfusion based cell culture system little optimization was required to match fed batch yield. Dr. Coffman also explained the advantage in downstream purification. Typically with fed batch all the processing must be done in one day, yet with perfusion they were able to process over 6-12 days thus allowing for smaller downstream columns. The smaller columns provided more cost effective purification in a smaller footprint. They also found that product quality was acceptable and not much different from their fed-batch process. Using this process, they were able to generate a kilogram of material using 100 liters in just 20 days. The process required less capital investment and operating expenses as it was highly automated.
While the results they got were very impressive, there were still issues to resolve including verifying continuous virus inactivation to 5 logs, identifying methods for verification of a closed system with the use of disposables, and on-line or at-line bioburden and endotoxin monitors were needed. Furthermore they needed to optimize the media to reduce the volume being used and to find the optimal mix of classic and concentrated media to provide the best productivity at the lowest possible cost. Despite the challenges, the positive results justify additional steps to solve these problems and further implement of this process.
Single Use Systems
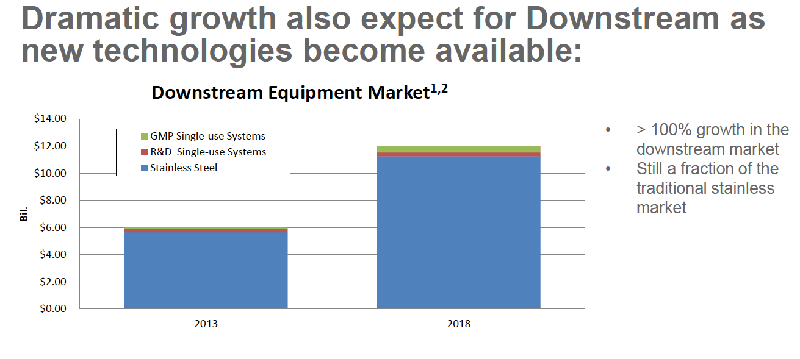
Single-use systems are increasingly being utilized to address many of the challenges facing biopharmaceutical manufacturing of the future including the need for flexible, multi-use, multi-product facilities. Growth of single-use systems in GMP facilities has been slow but steady. (Figure 1) Dramatic growth is also expected as more downstream single-use products become available. (Figure 2)
Figure 1
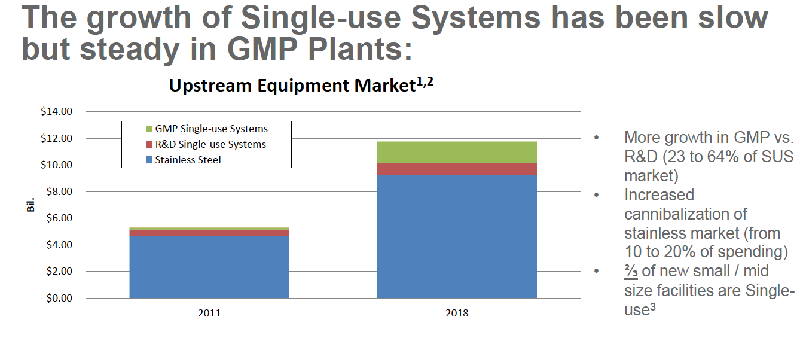
Figure 2
There are several reasons companies decide to move to single-use, multi-product facilities. As discussed earlier there is an increasing need to manufacture several products in the same facility due to improved yield and lower product volumes. In addition, emerging markets are utilizing single-use technologies to get facilities up and running faster and to simplify manufacturing operations. Single-use also enables a shorter time to market and reduced capital and operating costs.
Bioprocess Automation and IT Integration into Manufacturing Processes
As single-use facilities provide many benefits, they also present some areas of risk. Marc Puich, Vice President, Strategic Program Management at Werum IT Solutions identified some factors that led to increased risk:
- Increased manual activities means increased possibility of human error
- Increased complexity of consumables creates difficulties with genealogy and material tracking
- Designed for increased flexibility also increases possibility of human error and difficulties with IT efficiency
- More material “in space” creates difficulties in material tracking
Automation and implementation of a Manufacturing Execution System (MES) can mitigate the risks, thus providing a strong argument for implementation of MES in biopharmaceutical manufacturing. Not surprisingly, growth of MES in biophrarmaceutical manufacturing has been steady with more than 70% of top companies establishing global MES programs.
Shawn Opatka, Process Management, Emerson discussed the challenges involved in creating an MES for biopharmaceutical manufacturing. How do you build an MES recipe for an unknown product or make different products without having to change the MES recipe?
He then described the partnership between Emerson and Finesse in creating SmartMES. The goal was to provide a convenient and complete MES solution for the biopharmaceutical industry, thus making MES available for small to midsize biotech companies.
SmartMES is built is built on Emerson’s SynCade platform and provides the following benefits:
- Flexible unit based recipes
- A single recipe produces several products
- Offers a pre-configured solution for single-use bioprocess
SmartMES can also be paired with Finesse’s SmartFactory process. This allows for integration of all unit operations into one network that optimizes resource utilization and generates batch reports. Smart factory with Smart MES is a pre-validated, skidded solution that is production ready and ERP capable.
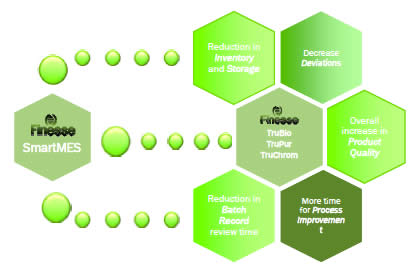
Additional advantages of the system include:
- The ability to stop deviations before they occur
- Ensuring correct resources are involved, i.e. people, equipment, equipment status, material documents, and work instructions
- Electronic batch record
- Implementation of release by exception
SmartMES is the first MES system pre-configured for the entire bioprocess train. It is designed to reduce cost and time deployment. Finesse can work with existing equipment and processes from a variety of suppliers as part of their SmartFactory system and they also have their own SmartSystems and SmartParts as well.
For further information, you can find Marc Puich’s presentation and Shawn Opatka’s presentation available for download here.
Outsourcing Projects to CMO/CDMOs
Companies need to consider whether they want to do the manufacturing in-house or outsource projects to a contract manufacturing organization (CMO) or a contract development and manufacturing organization (CDMO). Many CMO/CDMOs are one-stop shops where companies provide the protein and the CMO/CDMOs conduct the development and manufacturing work.
Chris Chen, Ph.D., Senior VP and CTO, WuXi AppTec discussed WuXi’s progress in building the largest single-use facility. The facility will be used for contract development and manufacturing. In the facility, they are establishing next generation platforms including an integrated continuous process for upstream and they are also working on doing the same for downstream.
One advantage that many CMO/CDMOs can offer is a reduced timeline. Dr. Chen stated, “of the last 31 breakthrough designation products WuXi touched 24 through work with biotech companies and entrepreneurs.”
WuXi is also embracing some of the novel therapeutics. They contract manufacture antibody drug conjugates (ADC) and have partnered with Ambrx. They are able to address all ADC manufacturing needs in separate facilities within 100 miles of each other. They also have a cell therapy manufacturing facility under construction in Philadelphia with 45,000 square feet of operational space. The facility will be complete at the end of 2016 and it is already fully booked.
Conclusion
The combination of mounting pressure to reduce drug prices, be the first to market, move into emerging markets and the introduction of novel therapeutics require manufacturing to constantly evolve to address the industry’s needs. The implementation of next generation technologies including perfusion, continuous processes, bioprocess automation and the increased use of single-use systems provide some of the advancements needed to respond to these manufacturing challenges.
The use of these new technologies in a variety of applications provides both the opportunity to learn new strategies for biopharmaceutical manufacturing and, as this conference was proof of, it also provides an opportunity to educate others on new ways to implement these technologies. For example the establishment of SmartMES and the SmartFactory as a way to implement bioprocess automation in a simplified, pre-configured system will further enable bioprocess automation and implementation of MES in biopharmaceutical manfuacturing.
However, there is still room for improvement. I heard repeatedly that improvements in both downstream single-use components and continuous processes are in development and are very desired.
