
Cadence Single Pass TFF Provides in-line Concentration, Enables Continuous process
Background
The concentration of biological drug substances may be necessary at various stages in the drug manufacturing process. Examples include post harvest, pre-capture or in-process volume reduction. Concentration may also be needed between chromatography steps.
The most widely used method for concentrating biologics is tangential flow filtration (TFF). In this method, the feed stream passes parallel to the membrane face as one portion passes through the membrane (permeate) while the remainder (retentate) is recirculated back to the feed reservoir. When the desired concentration is reached, the process is stopped. TFF is an efficient means for concentrating biologics, however sensitive proteins may have a problem with the recirculation of process fluids and aggregation or product degradation can occur.
Single Pass TFF (Tangential Flow Filtration)
One solution to the stress of recirculation is to implement Single Pass TFF (Tangential Flow Filtration). Single pass TFF provides an attractive alternative to traditional TFF as it uses a single pass flow through design, thus eliminating the recirculation loop. As a result single pass TFF permits high concentration of shear-sensitive or aggregation sensitive substances.
Cadence In Line Concentrator (ILC)
To make implementation of single pass TFF simpler and more efficient, Pall recently launched the Cadence In line Concentrator (ILC). The Cadence ILC was specifically designed to allow volume reduction before or after many different types of downstream processing steps. While it enables continuous processing by providing an easy to implement, plug and play design for coupling of different downstream processes, it can also be used as a stand alone system.
Key Benefits
Increased capacity and reduced costs
The Cadence ILC permits concentration of product before or after other downstream processing steps. As a result the overall process can be fully optimized to eliminate or reduce the need for in-process pools and can help to eliminate or reduce the size of intermediate storage tanks and the need for tank cleaning. Furthermore, Cadence ILC modules can be reused several times with minimal change in performance, thereby reducing consumables cost.
Optimize processing of highly shear-sensitive products
As discussed earlier, sensitive products may have difficulty in a traditional TFF system due to the recirculation of process fluids. With the Cadence ILC, substances only pass through the pump and module once, thus reducing residence times and shear exposure. Additionally, for pump sensitive products, by using pressurized vessels to flow the fluid through the module, the pump can be removed. Likewise, by eliminating mixing or foaming issues, potential product damage or aggregation can also be reduced.
No holder required
Cadence Inline Concentrator modules are provided assembled, and the cassettes and manifolds are pre-torqued between two end plates so that no extra holder is required.
Easy to implement
The Cadence ILC comes preassembled and only requires the connection of its feed port to a pressurized feed source. It is designed to be plug and play and easily integrates with other systems. Different module sizes can be used for different phases in the drug development cycle to provide scalability. The system can be used for post harvest, precapture volume reduction, in volume reduction, concentration between chromatography steps, and in some cases the final concentration.
Performance
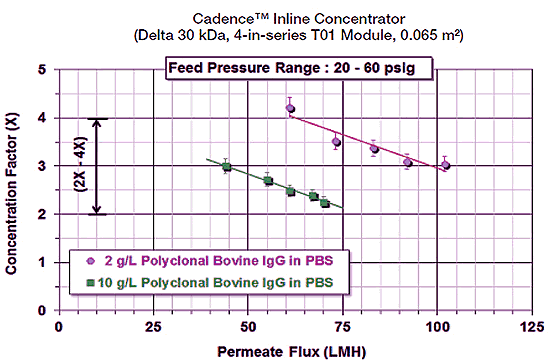
Depending on the initial concentration and product characteristics, a volume reduction of 2 to 4X (or higher) can be achieved with with Cadence ILC. Performance is further enhanced by coupling the proven high flux, high selectivity, and low protein binding attributes of Delta’s regenerated cellulose membrane with the Cadence ILC.
Figure 1:
Enables Continuous Process
The Cadence ILC permits in-process concentrating, which benefits continuous process applications such as perfusion culture methods. Concentration of bioprocess fluids with lower protein titer, such as those generated during perfusion, before multi-column or continuous chromatography enables significant process cost reduction. Continuous processing is also enabled by the easy integration of the Cadence ILC and its ability to couple a variety of processes for overall process integration.
For more information, please see the video: – www.pall.com/main/biopharmaceuticals/literature-library-details.page?id=20120625141616
