
Scalable Protein A Chromatography for High-Throughput Process Development
Process development is a critical part of biomanufacturing, but it can be very time and resource intensive. With recent industry initiatives around speed to market, process development is an area that could really benefit from high throughput solutions. One high-throughput process development tool for chromatography is the use of 96-well plates. These plate platforms permit automated screening of large numbers of conditions very efficiently and use only a small amount of material for testing. This platform is great for screening but requires bridging experiments to translate results to process scale. In addition, automating the 96-well process requires investment in liquid handling equipment in order to reach the full potential of the platform.
In a recent paper, “Applications of directly scalable protein A chromatography for high-throughput process development” authors, Scanlon et. al present two research-scale units designed to enable high-throughput process development for chromatography. The units in this study are based on established protein A and ion exchange ligands that are immobilized onto cellulose fiber absorbent mounted in poly-propylene housing forming what we henceforth call Fibro units. The paper by Scanlon et al. was published together with a group of expanded abstracts covering talks presented at the fourth High-Throughput Process Development (HTPD) Conference in Toledo, Spain.
The paper describes two case studies where authors demonstrate how the use of Fibro units functionalized with protein A at 0.4 mL laboratory-scale and 60 µL 96-well plate format can be used complimentary. The first case study involves the process development of a primary capture step on an industrially relevant mAb, varying process conditions such as the pH and concentration of elution and post-load wash buffers as well as flow rate. The second illustrates the integration of the capture step into upstream development by using the robust Fibro matrix to handle centrifuged material from ambr 15 bioreactors to obtain samples for analysis during a cell line selection screen.
In the first case study, elution peak and % recovery is examined using experimental conditions including pH and concentration of post load wash and elution buffers. The system demonstrated that this was a powerful high throughput tool that could be used to screen many experimental conditions with little time or material. Authors point out that a duplicate run of the 64 experimental conditions only took 4.5 hours. As a result, they were able to determine key conditions for further process optimization with the Fibro units that are scalable for industrial processes. (Figure 1). In this study a Fibro unit functionalized with protein A, 0.4 mL matrix volume was used.
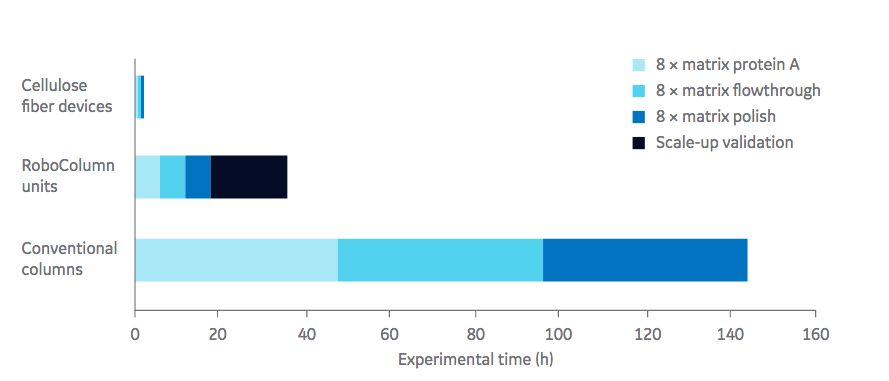
for conventional bead chromatography, eight parallel RoboColumn™ units, and Fibro units.
Authors explain how the Fibro absorbent material is designed to operate in a rapid cycling mode, with each unit capable of being cycled > 100 times in a single working shift. The units in development currently scale up to units capable of purifying kilograms of product in industrial processes.
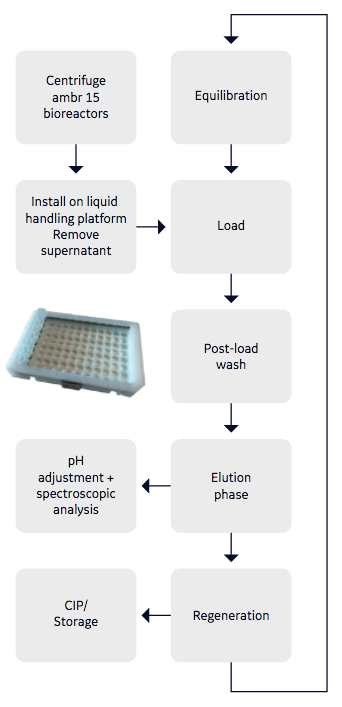
In the second case study, authors looked at a high-throughput solution for purifying unfiltered centrifuged supernatant from 48 x ambr™ 15 bioreactors to generate purified IgG. The purified IgG would be used to evaluate the performance of the different bioreactors and this information would be used in cell line selection. Authors used a bespoke 3D printed carrier for benchtop centrifugation, then bioreactors were transferred to the liquid handling deck where supernatant was aspirated from each bioreactor via liquid handling and was loaded in parallel sets of 8 individual chambers of the customized 96-well Fibro plate. Figure 2 provides the process flow diagram. In this study, Fibro matrix functionalized with protein A was used in a customized 96-well plate format, holding 60 µL Fibro matrix per well.
This process demonstrated high-throughput results with samples purified, with concentrations determined and pH adjusted in less than two hours.
In both studies, authors demonstrated the utility of Fibro technology as a tool for high throughput process development. Key advantages for the independent Fibro units connected to ÄKTA™ automated chromatography system includes the opportunity to generate large amounts of data and full chromatograms since a single chromatography cycle is completed within a few minutes. This enables for rapid bridging from HTPD to process scale.
The 96-well format has the advantage of needing even less sample material than the 0.4 mL Fibro units connected to ÄKTA automated chromatography system.
I was fortunate to be able to speak to one of the authors, Chris Morris, GE Healthcare, about the two case studies described in the paper. Below is a transcript of our interview.
What do you see as the areas where high-throughput process development can make the biggest impact in reducing process development time?
Time to market for a drug and robustness of process remain critical considerations for any drug manufacturer. Whether the technology here is used for rapid process development of an identified target or as a tool for looking at a larger number of clones during the selection phase, the ability to carry out these efforts in a fraction of the time is very attractive. It could also allow for a greater justification of going off platform with a new process, being able to start with a clean slate allowing for a fresh look at processing approaches to optimally produce and purify a new product, yet achieving the same level of process robustness all within existing time constraints.
What kind of impact can high-throughput tools have on overall process development beyond reducing timelines?
In a world where data is fast becoming king the need for better process understanding is clear. With speed comes an increase in number of experiments, and thus process understanding and robustness. As the industry moves to more complex targets where platformability becomes more challenging this is only going to continue to grow in importance.
In the second case study you used a high-throughput chromatography platform to inform cell line selection. In what other ways can these high throughput tools be combined to inform both upstream and downstream processes?
The rapid cycling technique allows for single-use operation which in turn offers future possibilities for in-line analytics or PAT – process analytical technology. Also, early synergy between USP and DSP means cell line selection can be based upon not just titer, but product quality, process productivity, and long term manufacturability.
What do you recommend for groups that don’t have access to liquid handling platforms, are there other ways high-throughput can be incorporated?
This was the precise intent of this project. The 0.4 mL LabScale Fibro units used here link to ÄKTA liquid chromatography systems, and due to the speed with which they can be run, they actually outpace the parallelized liquid handling platforms under more directly scalable conditions and yield much more data enabling better process understanding and future robustness.
What do you see as the biggest challenge of implementing high-throughput process development?
There are a number of challenges with traditional HTPD:
- Confidently linking small scale studies to process relevant scale operations, defining a scaling parameter such as residence time is required along with bridging studies.
- Training, experimental design, investment in equipment and appropriate models which have been tested.
- The burden HTPD places on analytics, where the bottleneck in throughput is shifted rather than removed.
These points are a further justification for the use of the LabScale Fibro units with ÄKTA chromatography systems as the data becomes directly scalable right up to full scale industrial processing, the units are simple to use with infrastructure that is more readily available, and additional automated analytics can be carried out by integrated software (UNICORN™) that allows the user to hone in on key variable samples where further offline analysis may be desired.
Header photo courtesy of GE Healthcare Life Sciences. Fibro technology utilizes the high flow rates and high capacities of cellulose fiber. Fibro chromatography enables fast mass transfer by convective flow while process flow is only restricted by the size of the pores in the matrix material.
Please see related articles:
- High Throughput Process Development in Biomanufacturing – Current Challenges and Benefits
- Utilizing High-Throughput Process Development Tools to Create a Purification Process for a Biosimilar Molecule
- Implementing Digital Biomanufacturing in Process Development
