
Validation of Virus Filtration in Continuous Bioprocessing
Adoption of continuous processes certainly seems on the biomanufacturing horizon and this is not surprising considering the benefits of an integrated continuous approach. Demonstrated benefits include operational flexibility and efficiency, product consistency, increased quality assurance, and cost savings. However moving toward continuous bioprocessing requires the development of enabling technologies to support this type of operation.
When using continuous bioprocessing applications for monoclonal antibody (mAb) or recombinant proteins, one critical consideration involves assurance of viral safety. While robust virus clearance is well understood for batch processes, many questions remain on how to implement viral safety into continuous bioprocesses. For example, use of virus filtration in continuous bioprocessing is likely to involve low flow rates, and significantly extended processing times compared to current batch applications. Successful implementation will require a very thorough understanding of the virus filter design space.
Further, validation of virus filters for continuous bioprocessing will require innovative test design to be able to demonstrate robust viral clearance, while also simulating extended filtration times and low flow rates, often considered worst-case parameters for validation of virus filters in a batch mode.
A recent poster by Pall Life Sciences, “Consideration of Filter Design Space for Validation of Virus Filtration in Continuous Processing Applications,” presents data to show some of the process inputs that should be evaluated to determine critical control attributes for continuous bioprocessing applications. The author, Dr. Nigel Jackson does a nice job discussing how a robust virus filter design space is required for implementation into continuous bioprocessing applications and key considerations required for success. He also presents data to show that they were able to achieve over 7 logs of virus clearance a simulated low flux continuous bioprocess.
Highlights
Dr. Jackson begins with an explanation of filter design space and then provides a nice graphic that gives a visual of the process involved in building a virus filter design space (Figure 1).
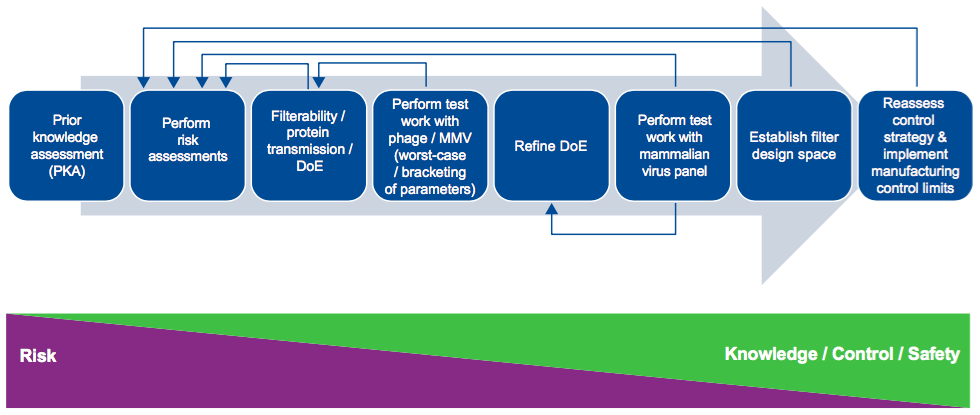
From here he presents data to show some of the process inputs they evaluated in determining critical process parameters for continuous bioprocessing. These included volumetric throughput, protein purity, protein aggregation, pH/Ionic strength, operating pressure/flux processing time, and process interruptions.
Next, the author discussed considerations important in creating a new continuous design space. Key points to consider include:
- Lower flow rates throughout the continuous process require virus filtration at lower operating pressures
- Significant operational and risk advantages to reduced filter replacement and long processing times
- Prior knowledge: low pressure or flow is now considered worst-case and must be validated
Last, Dr. Jackson provided results of a process simulation where the Pegasus Prime virus membrane’s robustness in the new continuous design space was tested. Results show that in the simulation they were able to achieve over 7 logs of virus clearance a low flux continuous bioprocess (Figure 2).
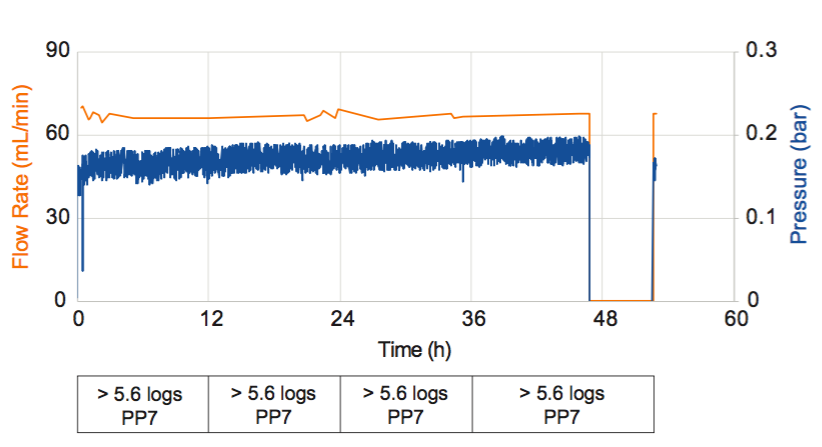
For full data and additional details, please see the poster reformatted in letter layout for easier reading
For more articles on continuous bioprocessing, please see:
https://downstreamcolumn.com/look-current-state-continuous-bioprocessing/

