
Viral Filtration Validation Study Design – A Systematic Method for Choosing the Most Appropriate Test Approach
Viral filter validation is important to ensure the removal of viruses under the conditions seen at process scale when using scale-down models. When designing an effective study, there are key components that must be decided on. First, it is important to select a virus spike level that will allow for quantification of virus removal, without having a negative impact on the scaled down process. Second, it is important to understand how a virus prefilter will be incorporated into the study design.
I recently read a set of application notes in which Pall Biotech shares their experience validating the Pegasus™ Prime Virus Removal Membrane Filters and the Pegasus™ Protect Virus Filters. In the note, they address both the issue of spike selection and incorporation of virus prefilters into study design. I found the included decision trees to be very helpful as well.
Highlights of the application note are below:
Selection of a Virus Spike Level
Selecting the correct virus spike level is critical to the success of a validation study. Over-spiking risks introducing contaminants that would normally not be present at this stage in the process. Over-spiking also negatively impacts the filterability performance and reduces the accuracy of the scale down model.
The basis for spike selection is:
- Use the purest spike available (ultracentrifugation essential)
- Use the most sensitive assay technique (large volume assays)
- Spike only what is needed to measure your target log reduction value (LRV)
The application note offers a very useful decision tree for spike selection (Figure 1).
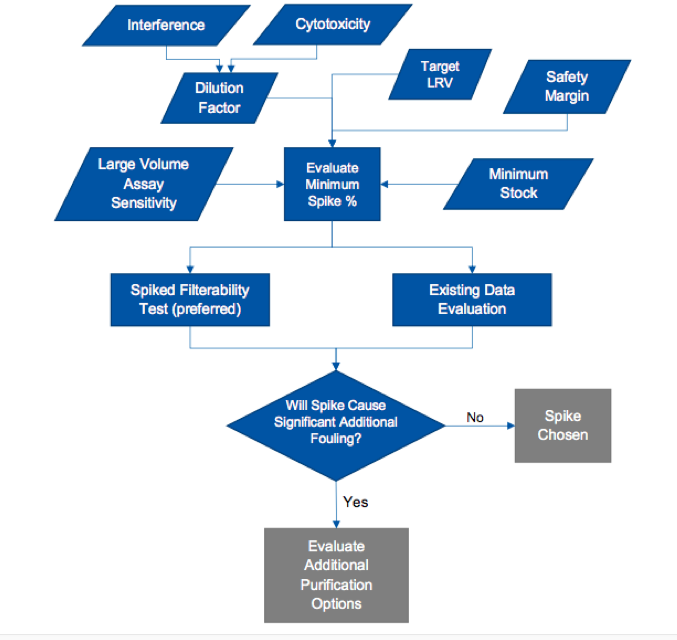
The decision tree supports a strategy of defining the minimum required spike and checking to ensure that the spike does not impact the filterability of the scale down model. An alternative approach, also outlined in the note, is to use existing data and experience to select a reasonable spike level known not to cause fouling in typical testing. However, this approach includes more risk to throughput. Also included in the note is a general guide as to what spike levels will have minimal impact on flow decay during a validation run using four viruses commonly used in validation testing.
It is noted that incorporating virus prefilters into your process can also significantly reduce spike related fouling for some viruses.
How to Incorporate a Prefilter
When designing a validation study, it is important to decide whether a prefilter is used and how it will be incorporated into the study design. The prefilter must be validated and qualified for virus removal. In the note, a decision tree is used to demonstrate different options used to validate virus filtration with an adsorptive prefilter (Figure 2).
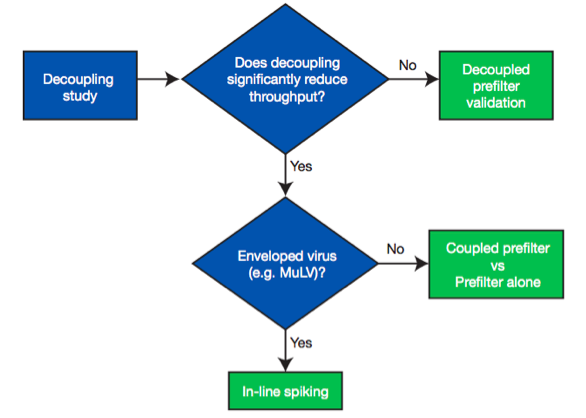
First, a decoupling study is recommended to determine the impact of prefilter decoupling on throughput performance before committing to a virus validation study.
Options include:
- Validation with a decoupled prefilter (the most preferred approach) – Test solution is prefiltered and the pooled, prefiltered test solution is spiked with virus before challenging the test virus filter alone.
- Validation with a decoupled prefilter (the most preferred approach) – Test solution is prefiltered and the pooled, prefiltered test solution is spiked with virus before challenging the test virus filter alone.
- Coupled prefilter vs. prefilter only – The retention due to a virus filter alone can be evaluated by comparing the coupled prefilter and virus filter compared to the prefilter alone. This method allows test solutions, which show significant decoupling effects to be tested for virus removal, but it will not work well for enveloped viruses.
- In-Line Spiking- In circumstances where decoupling of the prefilter reduces throughput significantly and the prefilter removes too much of the virus spike to implement coupled prefilter vs. prefilter only testing, in-line spiking is the best alternative. This approach resolves both decoupling issues and excess virus removal by the prefilter. The test solution is challenged to the prefilter and filter in series, but in between the filters, a virus spike is pumped in at a small percentage of the test solution flow and mixed before being challenged to the virus filter.
About the Filters:
Pegasus Prime Virus Removal Filters
Pall Pegasus Prime virus removal membrane provides robust virus retention to protect critical manufacturing bioprocesses, assure drug quality and safeguard health. Pegasus Prime virus filters combine high LRV with high throughput and high flow in mAb solutions to deliver an economic virus filtration solution with a small footprint for easy integration into single-use and automated processes. Pegasus Prime virus filters deliver a robust, high LRV independent of process parameters, process fluid and validation spike parameters.
Pegasus Protect Virus Filters
Pall Pegasus Protect prefilter membrane is tailored to provide protection to Pegasus Prime virus removal filters and to deliver robust filter performance in more challenging process fluids. The combination of Pegasus Protect and Pegasus Prime filters increases throughput and leads to a reduction in processing costs by a reduction in virus filter sizing and processing times.
To learn more, please see Virus Filtration
Application Notes:
- Validating Pegasus™ Prime virus removal membrane filters: How do I incorporate a prefilter in my virus clearance study?
- Validating Pegasus™ Prime Virus Membrane Filters: What Virus Spike Should I Use?
