Implementing Process Analytical Technology (PAT) in Continuous Bioprocessing
Continuous bioprocessing presents many exciting opportunities for improving biomanufacturing. Frequently discussed are methods for incorporating Process Analytical Technology (PAT) initiatives into a continuous operation. One advantage of implementing PAT in continuous bioprocessing operations is the opportunity to not just analyze what has happened, but to make real-time process control adjustments for increased quality assurance and greater process efficiency.
I recently saw an interesting poster, which addressed the implementation of PAT in continuous bioprocessing. The poster, “Process Analytical Technology (PAT) For Continuous Bioprocessing,” presented by Edita Botonjic-Sehic, Pall Corporation, discusses key steps in implementing PAT and enabling on-line and at-line real time continuous monitoring and automation.
Poster Highlights
The author begins by discussing the use of PAT to set monitoring and control strategies and summarizes a major difference between PAT in continuous bioprocessing and batch operations.
“In contrast to batch processing, in which local control of each piece of equipment is inmany occasions considered sufficient, in continuous manufacturing not only is local control mandatory, but also the entire process flow must be coordinated and equipped with second-level control systems that supervise and align the work of individual unit operations.”
Botonjic-Sehic, goes on to discuss how the selection of appropriate PAT tools is a crucial step toward setting efficient monitoring and control strategies for continuous processes.
Tools should include the following attributes:
- Easy-to-use instrumentation
- Capacity for measuring frequencies
- Ability to monitor multiple process parameters
- Directly measure Critical Quality Attributes (CQAs)
- Capture the real-time process state
- Eliminate traditional off-line techniques and increase efficiency.
The author then discusses the type of culture data that could be collected and trended in real-time via PAT tools in the future. Key critical quality attributes that could be measured include: bioburden levels, product concentration, host cell protein (HCP), aggregates, glycosylation profiles, metabolites, etc. In the poster, examples of percent aggregation, protein concentration and host cell protein were collected and monitored during various process steps and times. In Figure 1, I have included the host cell protein data.
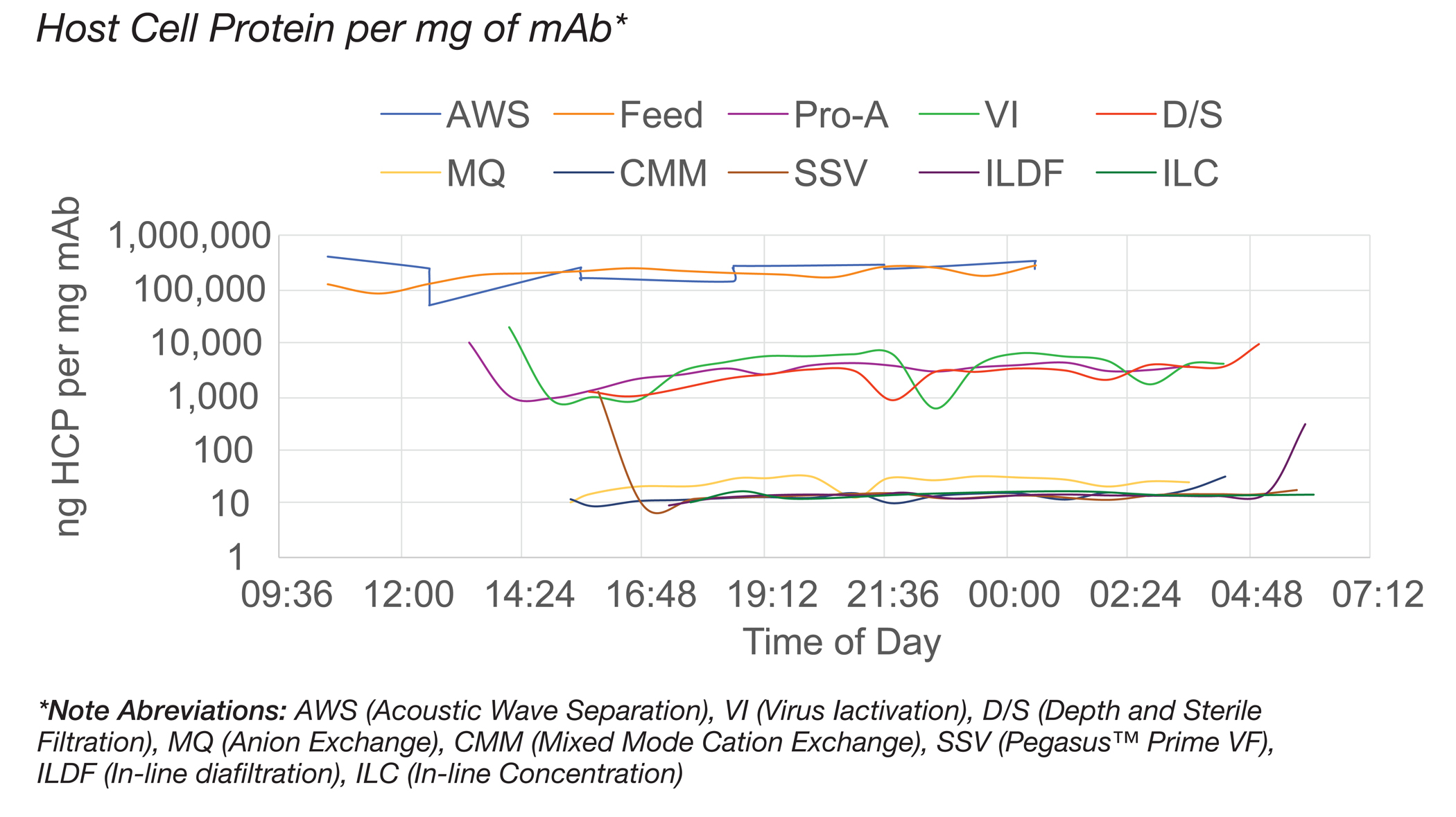
The author goes on to review how real-time data collection can assist in the development of chemometric models. Chemometric models can be used for predictive estimation of properties of a process and thereby help in process analysis, optimization, monitoring and control.
Conclusion
It has been well documented that implementing a PAT approach provides significant benefits in biomanufacturing. In fact, a couple key benefits of continuous bioprocessing naturally align well with process analytical technology (PAT) initiatives, for example, more consistent processes and product quality. However, PAT requires the development of technologies that can provide efficient on-line/in-line analyses and incorporation of these analyses into continuous bioprocesses is crucial. Therefore, it is important that we continue to examine the key areas of discussion presented by Botonjic-Sehic in this poster toward the goal of enabling on-line and at-line real time continuous monitoring and automation in a continuous bioprocess.
To see the full poster and data, please click the poster below:
*Pegasus is trademark of Pall Corporation
For Further Reading on Continuous Bioprocessing, please see:
- https://downstreamcolumn.com/validation-virus-filtration-continuous-bioprocessing/
- https://downstreamcolumn.com/continuous-biomanufacturing-implementation-now-and-in-the-future/

